





Arachnids
 |
 |
 |
 |
 |
 |
Almost anywhere one travels on Earth, some type of arachnid or chelicerate can be found. From deserts to tropical rainforests to deep-sea
hydrothermal vents, if you look closely enough, you are bound to find a
member of the Arachnida. Not only are theywidespread, arachnids are
also incredibly diverse, both in their number of species and their
ecology and behavior. So who are these creatures and why aren't more
people familiar with them? Below is a brief introduction to the major
groups of arachnids, with links to our research aimed at discovering
how these groups are related to one another.
Arachnids comprise the major lineages of chelicerates, which is one of the four major groups of arthropods. The other three major groups are insects, crustaceans (crabs and their kin), and myriapods (centipedes and millipedes). Insects are well known for their species diversity, but it is now recognized that mites may contain up to a million species (although most are not yet described). Spiders, too, have a rich fauna, with greater than 52,000 described species. The other orders of arachnids contain many fewer species, but harbor a great diversity of body forms, ecologies, behaviors, physiologies, and life histories.
Below, we follow the nomenclatural system for arachnid orders proposed by T. Savory (1972) in Systematic Zoology 21:122-125. A nice review of the less species-rich arachnid orders is: Harvey, M. 2002. The neglected cousins: What do we know about the smaller arachnid orders? Journal of Arachnology. 30:375-372.
(*Disclaimer* Any errors in the
text are entirely our own, and comments or corrections should be sent
to smasta@pdx.edu)
This material is based upon work supported by the National Science Foundation under Grant No. 0416628. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Taxonomy of Arachnida:
Phylum Arthropoda
Chelicerata
Pycnogonida
– Sea Spiders
Xiphosura –
Horseshoe crabs
Arachnida
Acari – (Acariformes and Parasitiformes) mites
and ticks
Amblypygi –
whip-spiders
Araneae –
spiders
Opiliones –
harvestmen
Palpigradi
Pseudoscorpiones
Ricinulei
Schizomida
Scorpiones
Solifugae – camel
or sun-spiders
Uropygi
or Thelyphonida –
vinegaroons
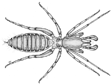
Below is a stylized body plan of a spider. Anatomical features
shared among arachnids include:
4 pairs of legs, which originate on the
cephalothorax (also called the prosoma)
1 pair of pedipalps
1 pair of chelicerae (from the Greek
“cheli” meaning claw)
Acari: Parasitiformes and Acariformes (ticks and mites)
The Acari (ticks, mites, and allies) exemplify diversity.
However, most species are tiny, even microscopic, and therefore often
overlooked. The largest species (such as velvet mites and ticks) are
generally not longer than 10 mm, while the smallest are tiny enough to
live in such bizarre places as the human hair follicle. The Acari are
commonly parasites of other organisms. For example, Locustacarus
buchneri is
a parasitic mite that lives in the tracheae (breathing pores) of Arctic
bumblebees, whereas other species specialize on marine gastropods. The
main lineages of Acari (the Acariformes, Opilioacariformes, and
Parasitiformes) have uncertain relationships, and some researchers
doubt they are very closely related. However, some
features that many Acari share include:
(1) A narrow anterior (gnathosoma or capitulum) with oral opening and
appendages. (2) Larvae with six legs (3) Anterior legs may be used in
sensing their environment.
The Acariformes lineage is the most species-rich arachnid group, with
over 30,000 currently described species (OConnor 1984). The
Parasitiformes includes the ticks and their close relatives. The Acari
have a worldwide distribution, living on plants and animals, and
inhabiting both freshwater and marine ecosystems. In fact, some mites
inhabit the area around deep-sea hydrothermal vents. In addition to
being diverse and filling many ecological niches, the Acari are
unparalleled among arachnids in their vast reproductive output (Walter
and Proctor 1999).
Lindquist, E.E. 1984. Current theories on
the evolution of major groups of Acari and on their relationships with
other groups of Arachnida, with consequent implications for their
classification. In: Acarology VI (Volume 1), D.A. Griffiths and C.E.
Bowman (eds), John Wiley & Sons, New York, pp. 28-62
OConnor, B.M. 1984. Phylogenetic relationships among higher taxa in the
Acariformes, with particular reference to the Astigmata. pp. 19-27 in
D.A. Griffiths and C.E. Bowman. 1984. Acarology VI, Vol. I.
Ellis-Horwood Ltd., Chichester.
Krantz, G.W. 1978. A Manual of Acarology. 2nd Edition. Oregon State
University, Corvallis.
Proctor, H.C. and I. Owens. 2000. Mites
and birds: diversity, parasitism and coevolution. Trends
in Ecology and Evolution 15:
358-364
Walter, D.E. and H.C. Proctor. 1999. Mites: Ecology, Evolution and
Behaviour. University of New South Wales Press and CABI Publishing, UK
Ricinulei (hooded tick-spiders)
"Two
features of the Ricinulei combine to make them the most romantic order
of the Arachnida, and might even support a claim to be placed among the
most absorbing orders of the animal kingdom...."
Theodore
Savory 1977 Arachnida, Academic Press, p 219.
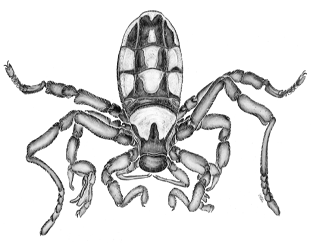
The two features that most fascinated Savory about ricinuleids
were their reputation for being extremely rare and the fact that they
were discovered in fossil form before a living specimen was found.
However, ricinuleids also have a number of anatomical and behavioral
features that make them quite intriguing:
(1) They are eyeless. (2) The anterior carapace forms a movable hood (a
“cucullus”). When the hood is lowered, it covers the mouth and the
chelicerae, which terminate in pincers. The female carries her single
egg under this hood, displaying a type of maternal care. (3) The young
hatch into six-legged "larvae" similar to mites and ticks. (4) The
pedipalps are small, and also end in tiny pincers. (5) In males, the
legs of the third pair are modified into copulatory organs, used in
transferring a spermatophore (see the illustration of the male
ricinuleid above, in which the third leg is bent forward to show these
organs).
Ricinuleids are an order of arachnids with few species, with only about 55
described species worldwide. They have been found only in a few scattered
tropical regions, and all extant species are currently placed into
three genera, Cryptocellus from
Neotropical America (Westwood 1874), Pseudocellus from
the Neotropics and Nearctic (Platnick 1980), and Ricinoides from
Africa (Ewing 1929). Ricinuleids are heavy-bodied arachnids with a
thick cuticle, and range in size from 5 to 10 mm. In fact, they have
been described as “the armored tanks of the arachnid world” (Platnick
2002). They feed on arthropods, and have a digestive tract similar to
other arachnids. The first ricinuleid was described from a
paleontological specimen by W. Buckland in 1837 (apparently he thought
it very similar to a weevil, as the abdomen is covered by a hard
unsegmented shield), the year before F.E. Guérin-Méneville (1938)
described the first extant specimen from Guinea (and erroneously
attributed the specimen to the arachnid order Opiliones).
Buckland, W. 1837. Geology and Minerology considered with reference to natural theology. Vol. 2. William Pickering, London.
Ewing, H. E. (1929). A syopsis of the American arachnids of the primitive order Ricinulei. Ann. Soc. Ent. Amer. xxii, 583-600.
Guérin-Méneville, F. E. (1838). Note sur l'Acanthodon et sur le Cryptostemme, nouveaux genres d'Arachnides. Revue Zoologique. 1:10-14.
Platnick, N. I. (2002). Ricinulei: In Amazonian arachnids and Myriapoda. Identification keys for all classes, orders, families, some genera, and a list of know terrestrial species. J. Adis (ed.) Pensoft Ed, Sofia-Moscow. P.381-386.
Platnick, N. I. (1980) On the phylogeny of Ricinulei. In Verhandlungen des 8. Internationalen Arachnologen-Kongress,Wien. J. Gruber (ed.) H. Egermann, Wien. Pp. 349-353.
 |
 |
 |
|---|
Harvestmen are often mistaken for spiders, given their
superficial eight-legged resemblance. However, they differ in
a number of ways. Harvestmen (also commonly called
daddy-longlegs) lack the silk glands that spiders possess, and
therefore cannot spin silk webs. They also lack the venom
glands that spiders possess, so even if you were to provoke a
harvestman enough for it to bite, it would result in little more than a
pinch.
While North American harvestmen are usually drab in color,
opilionids are quite rich in form. Their stout bodies may be
variously adorned with spines and contours while, depending on the
species, the legs may be short and robust or long and
graceful. Like most arachnids, harvestmen are nearly
blind. In general, opilionids have a pair of simple eyes set
atop a stalk (eye turret) on the top of their cephalothorax.
These eyes, however, are little more than light sensors and are
probably unable to focus or form images. Most of an
opilionid’s information about its surroundings comes from touch and
chemosensory organs. Unique among arachnids, harvestmen have
a pair of exocrine glands and can secrete a variety of compounds
including quinones, ketones, and phenols (Cokendolpher 1993; Gnaspini
1998). These and other chemicals are used by opilionids for
communication, defense, and even as antibacterial agents.
Harvestmen can be found in the dark and damp places. Living among leaf litter, decaying wood, and within stony crevices,
harvestmen are predators of many small invertebrates such as mites,
aphids, and caterpillars (Adams 1984; Allard 2005).
Researchers are still discovering surprising things about
harvestmen. In addition to their unique chemical secretions,
the sheer diversity and abundance of
these animals make them fascinating animals to study.
Adams, J. 1984. The habitat and feeding ecology of woodland harvestmen (Opiliones) in England, Oikos 42: 361-370.
Allard, C. and Yeargan, K. 2005. Effect of diet on development and reproduction of the harvestmen Phalangium opilio (Opiliones: Phalangiidae). Environmental Ecology 34(1): 6-13.
Cokendolpher J. 1993. Pathogens and Parasites of Opiliones (Arthropoda: Arachnida). Journal of Arachnology 21: 120-146.
Gnaspini, P. 1998. Chemical and Behavioral Defenses of a Neotropical Cavernicolous Harvestman: Goniosoma spelaeum (Opiliones, Laniatores, Gonyleptidae). Journal of Arachnology 26: 81-90.
For further reading, see:
Pinto-da-Rocha, R., G. Machado, and G. Giribet. 2007. Harvestmen: The Biology of Opiliones. Harvard University Press, Cambridge, Massachusetts.
( Robert Richardson, a graduate student in the Biology Department at PSU, was the primary author of this section.)Palpigradi (micro-whip scorpions)
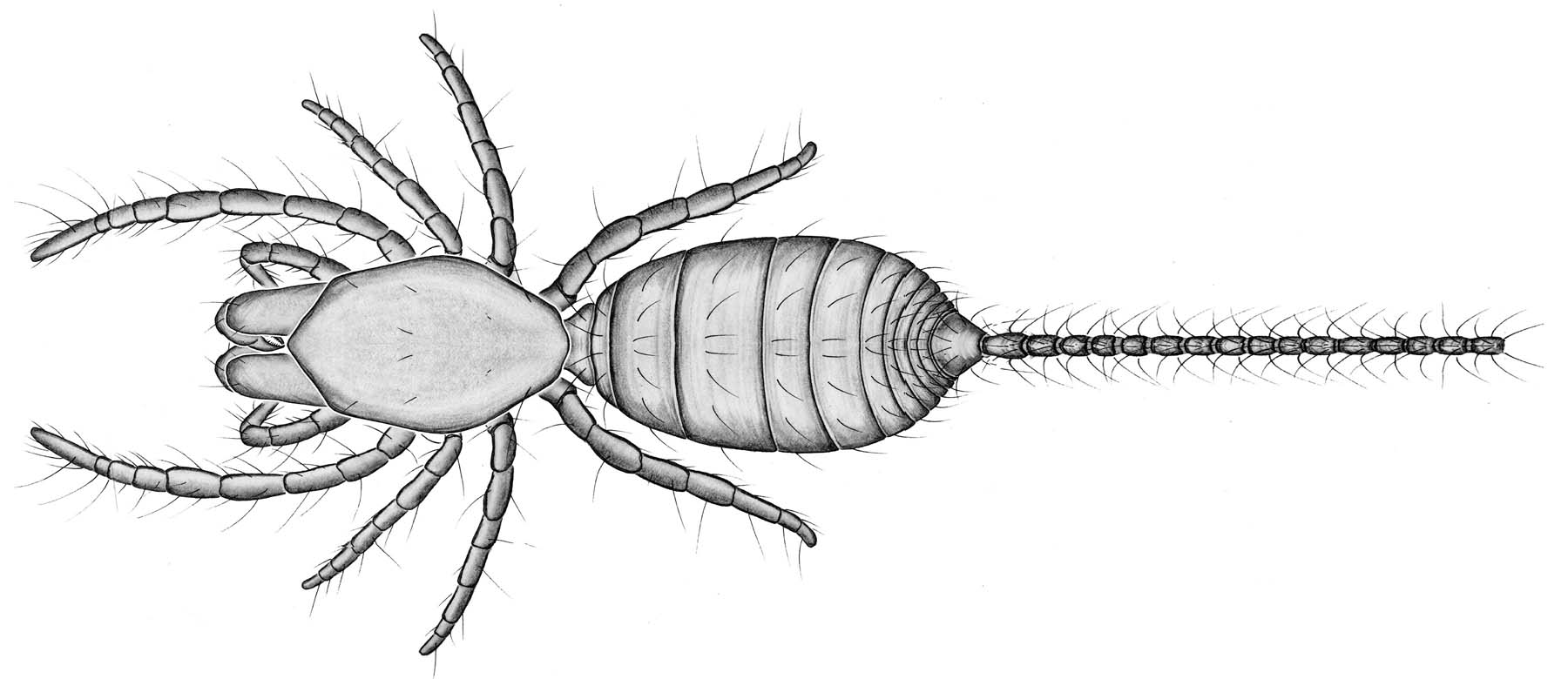
These tiny arachnids (less than 3 mm) generally live in soil
underneath rocks and stones where humidity is high. Very little is
known about their biology. Although little is known about their
reproductive behavior, it has been noted that only a few large eggs are
laid at a time. Other traits palpigrades exhibit include:
(1) Lack of eyes. (2) Thin and pale integument. (3) Cephalothorax is
divided into two plates between the 3rd and 4th legs. (4) Pedipalps are adapted for
locomotion only. (5) Segmented abdomen is broadly connected to the
cephalothora. (6) A moveable flagellum extends from the end of the
abdomen. (7) Some species have three pairs of book lungs; others lack
discrete gas exchange organs and probably respire across their cuticle.
About 80 species are described, from southern Europe, Africa, southeast
Asia, and the Americas. Most species have been described quite recently
(see Condé 1996). It has been suggested that palpigrades are not
completely terrestrial because the species Leptokoenenia
scurra from
littoral (seashore) regions is able to swim in seawater (Monniot 1966).
However, this conclusion is debatable, as palpigrades have sensory
trichobothria (hair-like structures) that function only in air (Walter
and Procter 1999).
Condé, B. 1996. Les Palpigrades,
1885-1995: Acquisitions et lacunes. Rev.
Suisse Zool., hors sér. 1:
87-106.
Hansen, H. J., and W. Sorensen . 1897 . The order Palpigradi Thorell (Koenenia
mirabilis Grassi)
and its relationships to other Arachnida . Entomol. Tidskr. 18: 223-240
.
Monniot, F. 1966. Un palpigrade interstitiel : Leptokoenenia
scurra, n. sp. Rev. Ecol. Biol. Sol. 3:41-64.
Walter, D.E. and H.C. Proctor. 1999.
Mites: Ecology, Evolution and Behaviour. University of New South Wales
Press and CABI Publishing, UK
Pseudoscorpiones (pseudoscorpions)

Pseudoscorpions are small arachnids (typically less than 5 mm in length). Their common name is “false scorpion,” as they look superficially like the larger true scorpion but lack the elongate metasoma (tail) and telson (stinger). They have long grasping pedipalps which they use to grab prey, such as springtails and mites. Many have bodies that are rather flat, which enables them to occupy small crevices. Here in the Pacific Northwest, they can be abundant in lead litter, but some species live under the bark of trees, under rocks, and some even live in caves.
Other interesting features that pseudoscorpions have is a silk-spinning apparatus in their chelicera. Unlike spiders though, their silk is not used to trap prey, but instead to create nests. Pseudoscorpions show highly developed maternal care, in which the female "nurses" the offspring. A female will lay eggs in brood sac and attach them to her underside. Some species of pseudoscorpions have highly complex courtship routines. Males attach a stalked spermatophore (sperm sac) to a substrate (Weygoldt 1969), which the female takes up as she passes over.
A few species (such as the neotropical Cordylochernes
scorpiodes) have been found to display an interesting
‘hitchhiking’ behavior, grasping onto other larger arthropods (such as
harlequin beetles) and catching a ride for free. It has been suggested
that this behavior, called phoresy, may enable individuals to move
easily into new areas of habitat (Zeh and Zeh 1992). Another species
(Paratemnoides elongatus) displays some degree of sociality, showing
cooperative predation and construction of communal molting nests (Zeh and Zeh 1990).
Chamberlin, J.C. 1931. The arachnid order Chelonethida. Stanford University Publication of Biological Science 7: 1-284.
Harvey M.S. 1990. Catalogue of the Pseudoscorpionida (Mahnert V, Ed.). Manchester University Press.
Harvey, M.S. 1992. The phylogeny and classification of the Pseudoscorpionida (Chelicerate: Arachnida). Invertebrate Taxonomy 6: 1373-1435.
Weygoldt, P. 1969. The Biology of Pseudoscorpions. Harvard University Press, Cambridge, Massachusetts.
Zeh, D.W., and J.A. Zeh. 1992. Dispersal-generated sexual selection in a beetle-riding pseudoscorpion. Behavioral Ecology and Sociobiology 30: 135-142.
Zeh, J.A., and D.W. Zeh. 1990. Cooperative foraging for large prey by Paratemnus elongatus (Pseudoscorpionida, Atemnidae). Journal of Arachnology. 18 3:307-311.
Solifugae (sun-spiders, camel-spiders, or wind scorpions)
“it may be true to say that Solifugae have the most formidable pair of jaws in the animal world”
(Savory 1964)
Solifugids (also called solpugids) are fast-moving arachnids
with a fearsome appearance resulting from their enlarged chelicerae
(jaws), which may be as large as the rest of the animal’s prosoma. Most
species are opportunistic generalist predators, although
a few species specialize on termites. The name of the order ‘Solifugae’
is derived from Latin — “those that flee from the sun” — and most
species are nocturnal. The smallest species measure only a few
millimeters in length, while the largest are about 10 cm (4 inches).
Some species are fast runners, attaining speeds up to 10 mph. Many
species construct somewhat permanent burrows, remaining inside for much
of the year, depending on rainfall patterns (Punzo 1998). Characteristics
include:
(1) Enlarged articulated chelicerae, often bearing teeth-like
serrations. (2) A rostrum, or beak-like mouth. (3) Long pedipalps, used
as sensory organs. (4) A reduced first pair of legs. (5) Mallet-shaped
sense organs called “malleoli” on the basal segments of leg 4, more
pronounced in males. (6) In males, heavily modified bristles (flagella)
on the fixed chelicerae-finger (except in members of the family
Eremobatidae).
There are just over 1,000 described species of solifugids, which are
found mostly in dry, warm tropical and sub-tropical regions worldwide
(except Australia). The only European species are found in southeast
Spain, while American species are mostly confined to portions of the
west coast of the United States, and the north and west coasts of South
America.
Brownell, P.H., & Farley, R.D. 1974. The organization of the malleolar sensory system in the solpugid Chanbria sp. Tissue and Cell 6(3): 471–485
Punzo, F. 1998. The Biology of Camel-Spiders (Aachnida, Solifugae). Kluwer Academic Publishers: Boston.
Savory, T. H. 1964. Arachnida. Academic Press, London
Uropygi or Thelyphonida (whip-scorpions or vinegaroons)
 |
|---|
The common name “vinegaroon” comes from the vinegar-smelling
acetic acid these animals secrete from glands when they are disturbed.
One species lives in the southern United States, and happens to be
quite large (up to 85 mm!), truly living up to its name, Mastigoproctus
giganteus. General features of uropygids include:
(1) One pair of eyes at the anterior of the cephalothorax, and three on each side. (2) A long
whip-like flagellum on the pygidium, a small plate made up of the last
three segments. The function of this structure is unclear, but it is
possible that it may be used to detect air movement, and/or may have a
chemosensory function. (3) Only six of the eight legs are used for
walking, as the first legs are elongated and held out horizontally as
the animal walks. These first legs function as sensory organs (this
similarity is shared by Schizomida, Amblypygi, and Solifugae). (4) Most
species have robust pedipalps, which are raptorial and move on a
horizontal (lateral) plane.
Over 100 species, both tropical and subtropical, occur in two separate
American regions (southern North America and northeastern South
America), as well as West Africa and eastern Asia. Males secrete a
sperm sac, which is transferred to the female. The gravid female burrows,
ceases eating, and lays multiple eggs within a mucous membrane. In the
initial stages after hatching, the young attach themselves to the mother’s back. In
Mastigoproctus, the young disperse after their first molt, and the
mother dies soon after.
Dunlop, J. and C. A. Horrocks. 1996. A new upper Carboniferous whip scorpion (Arachnida: Uropygi: Thelyphonida) with a revision of the British Carboniferous Uropygi. Zoologischer Anzeiger 234:293-306.
Haupt, J. and D. Song. 1996. Revision of East Asian whip scorpions (Arachnida Uropygi Thelyphonida) I. China and Japan. Arthropoda Selecta. 5:43-52.
Rowland, J. M. and J. A. L. Cooke. 1973. Systematics of the arachnid order Uropygida (=Thelyphonida). Journal of Arachnology 1:55-71.
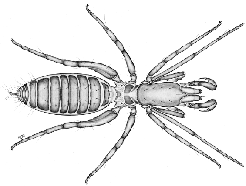 |
|---|
These small animals (less than 5mm) inhabit leaf litter and soil in the tropics and subtropics. These tiny animals lack eyes, and their first legs are modified as sensory organs, with which they sense their environment. Like the Thelyphonida, their pedipalps are well developed and raptorial, but unlike Thelyphonida, these move vertically. If you look closely, you may also note that their cephalothorax is divided into two plates.

Learn more about common spiders in the Portland, Oregon area at the Spiders of Portland website
 |
 |
|---|
Amblypygids are unusual looking arachnids, with an extremely long and thin pair of first legs. These look similar to whips, hence their common name “whip spiders”. These long appendages are used like feelers to sense their environment. Amblypygids often walk sideways with one whip-like leg held out in front of the body for sensing ahead of the animal, and the other held out to the side, sensing the adjacent surroundings.
Whip spiders are nocturnal and found in tropical and subtropical regions, hence few people in the USA are familiar with these arachnids. They are fairly large for a chelicerate, ranging from ~10 - 25 cm in size. Their body is flattened dorso-ventrally, which allows them to hide in small spaces such as under tree bark, in rock crevices, or in leaf litter.
Despite their common name, whip spiders differ from true spiders in a number of ways. Unlike true spiders, whip spiders do not possess silk glands or venom glands. They subdue their prey by means of long pedipalps that are modified into raptorial pincers. The pedipalps are covered with sensory hairs and are spiked along their inner edge, and end in a prehensile spiked grasping claw. Further details of whip spider biology can be found in Weygoldt 2000.
Weygoldt, P. 2000. Whip Spiders (Chelicerata: Amblypygi): Their Biology, Morphology and Systematics. Apollo Books, Stenstrup, Denmark.
 |
 |
|---|
Scorpions in your backyard! Yes, we have scorpions in Oregon! The resident scorpion species of the PNW is Uroctonus mordax, the Pacific Northwest Forest Scorpion (also called the California Forest Scorpion), as illustrated in the picture on the left. The Forest Scorpion can be found from California into southern Washington, and prefers to live and burrow in dark, damp environments such as under rocks and leaf litter. They are reddish-brown in color, can grow to about 3-5 cm in length, and feed on small insects and insect larvae. Unlike its more infamous Southwestern kin, the Forest Scorpion’s venomous sting is typically less painful than a bee sting.
Scorpions have the same general body plan shared by arachnids, with a few very recognizable modifications. The pedipalps are modified into large “pincers,” and are used for grasping food and in mating. The last segment of their large tail is the telson, and it contains the venom glands and ends in the characteristic barbed stinger, the aculeus. Scorpions have reduced eyes, and sense their environment via chemical and mechanical signals.
While greatly feared for their harmful sting, only a small fraction of scorpion species, (~ 25 species), are known to be lethal to humans, most of which are members of the Superfamily Buthoidea. Being predators, the function of scorpion venom is primarily to subdue prey. Venoms have been found to be prey-specific, varying in their potency and selectively for affecting different animals such as insects, small lizards, or mammals. Venom may also be used to deter a potential predator of the scorpion.
Scorpion venoms are cocktails of salts, enzymes, small molecules and proteins. Venom composition varies greatly across species, but most venom components act on the ion channels of nerve cells to paralyze prey. Recently, scorpion venoms have become an appealing research subject for their medical applications, with potential for treating cardiovascular and autoimmune diseases, as well as tumors and epilepsy.
Courtship and mating is behaviorally complex in scorpions. The ritualized courtship process begins when the male signals to the female by tapping against the substrate. Once the female is enticed, the male grasps her pedipalps in his own and proceeds to move the female around in a courtship dance, known as the “promenade à deux”. Males may also engage in a chelicerae “kiss” with the female, by grasping her chelae with his. The male moves over the substrate until he finds an ideal location for depositing his spermatophore directly onto the ground, and moves the female over it.
Also unusual amongst most arthropods, scorpions exhibit maternal care of their young, which are “born” live from the mother, rather than hatching from an egg. As the young are birthed one-by-one, they climb onto the back of the mother where they remain until their first molt. Further details of scorpion biology can be found in Polis 1990.
Polis, G. A. 1990. The Biology of Scorpions. Stanford University Press, Stanford, California.
(Molly Radany, a former graduate student at PSU, was the primary author of this section.)