![]()
![]()
Chapter 23 Workshop Problems
Polymers
1. For each of the following polymers, identify
a) the repeat unit
b) whether that polymer would be formed by chain-reaction or
step-reaction polymerization
c) the monomer(s) that would be appropriate for synthesis of
that polymer
d) whether that polymer would be characterized as an addition
or condensation polymer
e) the characteristic functional groups present in that polymer
f) the degree of polymerization that would correspond to a molecular
weight of 500,000
i) polypropylene
ii) Teflon
iii) Nylon 610
iv) cellulose
2. Styrene can readily be polymerized by strong acid, by reactive anions, or by a radical initiator. Give a reasonable mechanism for each process (use curved arrows to show movement of electron pairs or "fish hooks" to show movement of single electrons). You need to show just a few of the polymer chain lengthening steps. Explain why in each case the polymer has a regular (head-to-tail) structure with the phenyl group on every other carbon in the polymer backbone.
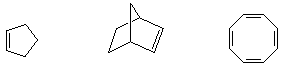
3. A relatively new method of polymerization of alkenes is called ring-opening metathesis polymerization (ROMP). It is based on the discovery that some transition metal complexes can complex divalent carbon and exchange ligands with an alkene, as shown below.
![]()
If the original alkene is part of a ring, show how this process could lead to polymer. Write the structure of the polymer that would be formed from ROMP of the following monomers: