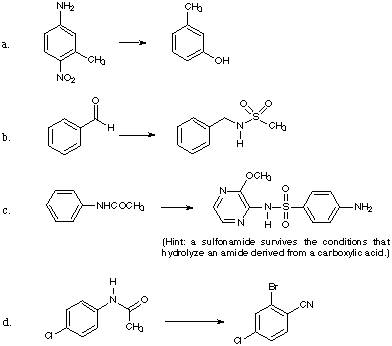
![]()
![]()
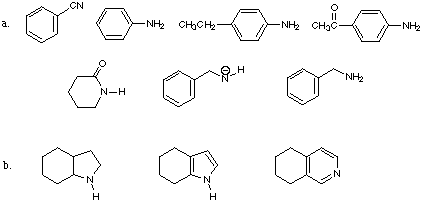
1. List the following ions and molecules in order of increasing basicity. Explain your choice clearly using words and structures (where appropriate).
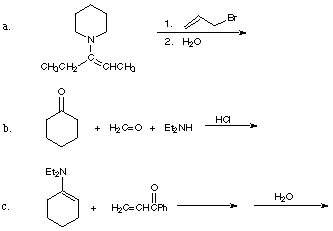
2. Give the product(s) and reasonable mechanisms for the following reactions.
3. Determine the structure of the following compound using the chemical and spectral evidence provided. This compound, C9H11N, gives rapid evolution of a gas when treated with aqueous nitrous acid at 0 °C. The 1H NMR spectrum exhibits a sharp singlet at 0.90 ppm (2H), a multiplet at 2.40-3.00 ppm (4H), and a multiplet at 7.1-7.3 ppm(5H). It exhibits the following 13C NMR spectrum: 12.9 ppm (t), 25.5 (s), 126.1 (d), 126.2 (d), 128.2 (d), 139.1 (s).
4. Show how the following conversions could be carried out
in three or fewer steps. Provide structures for each intermediate
that would be isolated and indicate reagents and conditions for
each step.