![]()
![]()
Chapter 18 Workshop Problems
Exam 3 Review
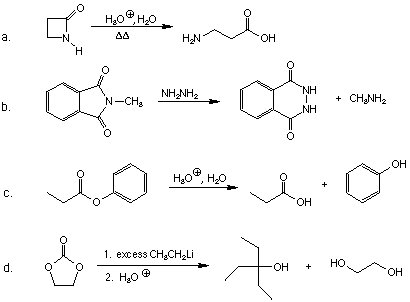
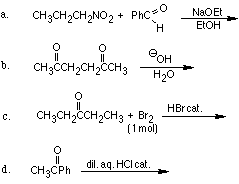
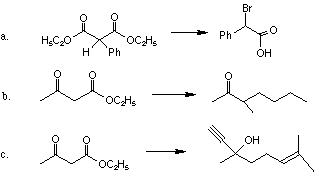
1. Give the products for the following reactions.
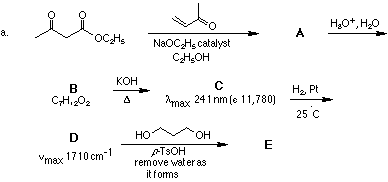
2. Provide structures for the lettered compounds in the following reaction schemes:
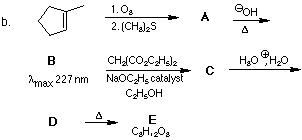
3. Provide structures that satisfy the following descriptions:
a. the compound, C6H10O3, that exhibits strong absorption in the infrared spectrum at 1754 and 1818 cm-1 and the following 1H NMR spectrum: 1.19 ppm (t, 3H) and 2.48 ppm (q, 2H).
b. the stereoisomer of 3-hydroxycyclohexanecarboxylic acid that could form a lactone.
c. the compound, C6H12O2, that would undergo reaction with excess ethyl magnesium bromide to give the compound shown below.
4. Show how each of the following transformations could be carried out in three or fewer steps. Show reagents for each step and structures of intermediate products.
5. Propose a plausible mechanism for each of the following reactions. Provide all important Lewis structures that contribute to the resonance hybrid for delocalized intermediates, and point out any important driving forces.