![]()
![]()
1. (4 points) Write the possible structures for the enolate (or enolates) that would be formed from the following. Write all resonance forms and remember stereochemistry if there are multiple possibilities.
a) 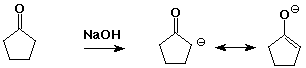
b) 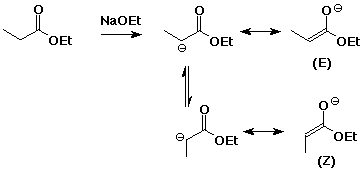
2. (2 points) Identify the starting compounds that would have been used in the condensation reaction that created the product below.
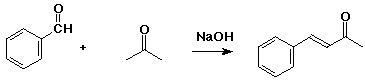
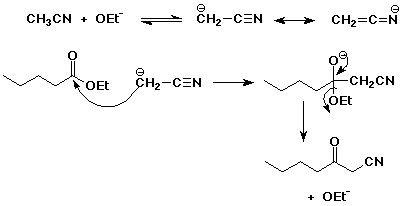
3. (4 points) The alpha-positions of nitriles can be deprotonated and act as nucleophiles. Show the resonance forms that are possible for the alpha-anion derived from acetonitrile (CH3CN), then write a mechanism for the reaction shown below.