![]()
![]()
A simple template for the active site of chymotrypsin is shown below. Chymotrypsin is a peptidase that specifically hydrolyzes a polypeptide next to (on the C-side) an aromatic amino acid (Phe, Tyr, or Trp). Use the template to follow the sequence of steps involved.
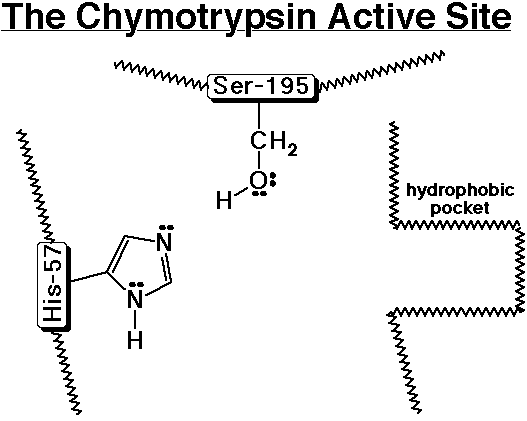
1) A polypeptide enters the active site and the aromatic amino acid side chain nestles into the hydrophobic pocket.
Nucleophilic substitution (2 steps)
2) Ser-195 acts as a nucleophile to add to the carbonyl group of the peptide.
SIMULTANEOUSLY, His-57 removes a proton from Ser-195 to make it a better nucleophile.
3) The tetrahedral intermediate splits out an amino group.
SIMULTANEOUSLY, His-57 donates a proton to the amino group to make it a better leaving group.
*******
4) The broken peptide fragment (the N-part) leaves the active site and is replaced by water.
Nucleophilic substitution (2 steps)
5) Water acts as a nucleophile to add to the carbonyl group of the intermediate (which is now an ester).
SIMULTANEOUSLY, His-57 removes a proton from water to make it a better nucleophile.
6) The tetrahedral intermediate splits out the Ser-195 group.
SIMULTANEOUSLY, His-57 donates a proton to Ser-195 to make it a better leaving group.
*******
7) The other part of the broken peptide (the C-part) leaves the active site and is replaced by another polypeptide (same as step1 above).