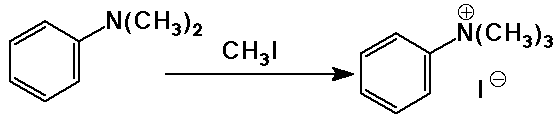Chapter 12 - Amines

Organic Compounds Containing Nitrogen
- a wide variety of functional groups, including combinations with oxygen

Classification of Amines
- primary (1°) - one C attached to N
- secondary (2°) - two C attached to N
- tertiary (3°) - three C attached to N
- quaternary (4°) - four C attached to N (and a positive charge)
- note the difference from other functional groups, which are classified
according to the C to which the functional group is attached

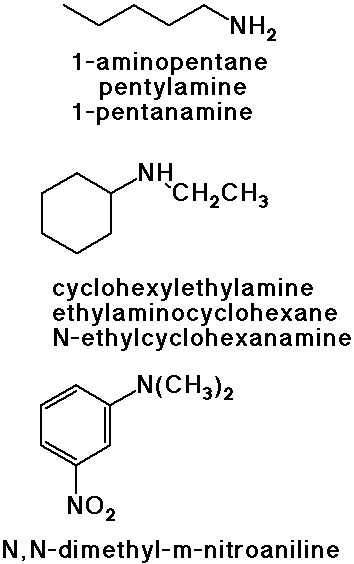
Nomenclature of Amines
- IUPAC: amino- substituent
- common: alkyl amine
- Chemical Abstracts: alkanamine
Structure of Amines
- sp3 (tetrahedral) nitrogen including the lone pair

- polar bonds make small amines water-soluble, good H-bonding
Basicity of Amines, Acidity of Ammonium Ions
- N lone pair relatively easily protonated
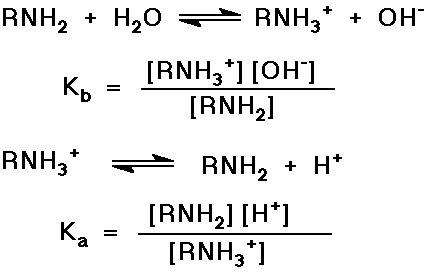
- note that Kb x Ka = [H+] [OH-]
= Kw = 10-14
or pKa + pKb = 14
- recall that when pH = pKa , there are equal concentrations
of the conjugate acid and conjugate base present (i.e., RNH2
and RNH3+ )
- for typical aliphatic amines, pKb = 3 - 4
so pKa = 10 - 11 for their ammonium ions
so at around pH 10 - 11 , RNH2 and RNH3+
are both present
- for typical aromatic amines, pKb = 9 - 10
so pKa = 4 - 5 for their ammonium ions
so at around pH 4 - 5 , ArNH2 and ArNH3+
are both present
- water solubility of amines can be easily changed with pH
aromatic amines are water-soluble (protonated) below pH 4
aliphatic amines are water-soluble (protonated) below pH 9
Basicity Trends
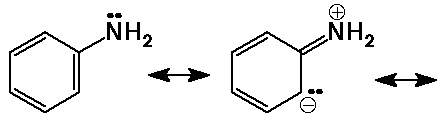
- aromatic amines are less basic due to resonance delocalization of the
N lone pair
- amides are nonbasic due to strong delocalization of the N lone pair

- electron withdrawing effects decrease basicity
because the N lone pair is less available for bonding to a proton
Optical Resolution
- a racemic mixture of a chiral amine can be separated into enantiomers
if a sample of a chiral acid is available
e.g., lactic acid or citric acid from natural sources are chiral
- the amine-acid salts (ammonium carboxylates) are diastereomers and
may have distinctive properties that would allow them to be separated (e.g.,
solubility)
Preparations of Amines
- substitution reactions:
SN2 reaction of ammonia on alkyl halides
but the amine product is still nucleophilic and further substitution often
results
primary amines can be made by using a great excess of NH3 to
avoid further substitution
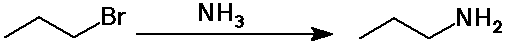

Reactions of Amines
- substitution reactions:
SN2 reactions on alkyl halides
but oversubstitution is a problem, except for making quaternary ammonium
ions
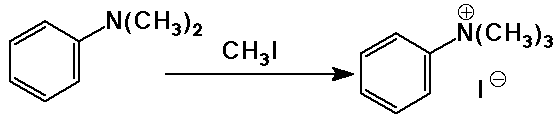
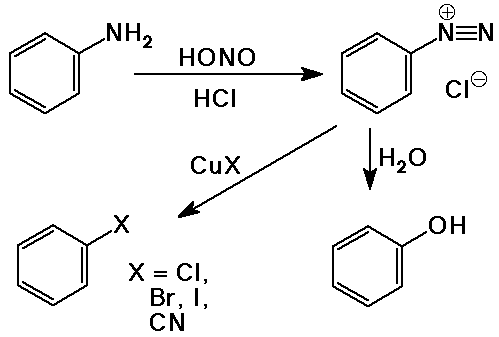
Diazonium Salts
- primary amines react with HNO2 (nitrous acid) in a diazotization
reaction
- aryl diazonium ions are relatively stable but can be replaced by many
nucleophiles
this method provides a way to attach nucleophiles to aromatic rings
(recall that most aromatic substitutions were electrophilic substitutions)
- Sandmeyer reactions - CuX as catalyst to convert diazonium ion to ArX
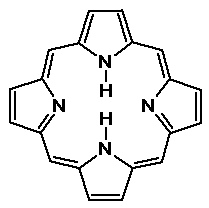
Pyrrole
- a 5-membered heterocyclic ring
- aromatic, because there are six pi electrons (including the N lone
pair)
pyrrole is not basic because the N lone pair is used in the aromaticity
- pyrrole is even more reactive than benzene in electrophilic aromatic
substitution

- the porphine ring system is a tetrapyrrole - found in heme, chlorophyll,
etc.
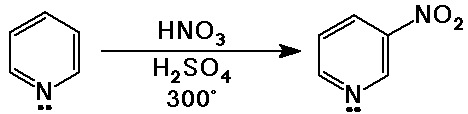
Pyridine
- a 6-membered heterocyclic ring
- aromatic, because there are six pi electrons (3 double bonds)
pyridine is basic because the N lone pair is available for bonding
- pyridine is less reactive than benzene in electrophilic aromatic substitution
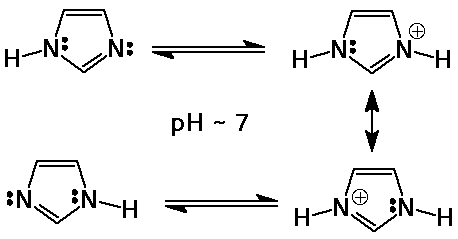
Imidazole
- a 5-membered heterocyclic ring with two N
- one N lone pair is availble for bonding (basic)
pKb for imidazole is close to 7
it is a common acid (and base) catalyst in biological systems around pH
7
Alkaloids
- naturally occurring amines, such as morphine
the alkaloid name comes from their basic (alkaline) properties
![]()
![]()
![]()




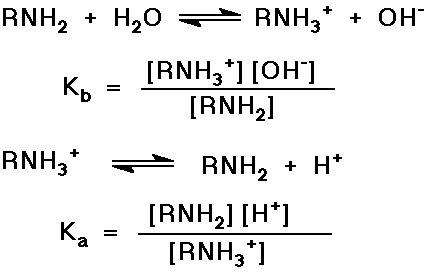


![]()