
Chemistry 332 - Spring 1996
Elements of Organic Chemistry II

Homework, Chapter 16 - Lipids and Nucleic Acids
McMurry, pp 504-506:
Problems 16.23-25, 31-33, 36-40, 42, 43, 47
1. Find the structure of olestra, the new synthetic fat substitute. Its
special property of being apparently undigestible is that it doesn't hydrolyze
readily, even in stomach acid. But if it did hydrolyze completely, what
products would you expect? How would this affect its nutritive value?
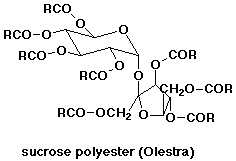
Olestra has a mix of fatty acids attached to the OH groups of sucrose
(typically 6 - 8 fatty acids). This resembles the usual triglyceride structure
for fats, so it has a similar "mouth feel", and it behaves similarly
in cooking - actually even less likely than fats to break down on heating.
If our digestive enzymes could break it down, it would give sucrose (and
then glucose plus fructose) and 6 - 8 fatty acids. Per mole, it would contain
more energy than a typical triglyceride because it has more fatty acids.
Per gram, however, it would be about the same as fats, since the great majority
of the weight in both molecules is the fatty acid chains.
The concern about Olestra is that it may act as a nonpolar, water-insoluble
medium that absorbs other important nonpolar nutrients (especially Vitamins
A and K) and escorts them out of the digestive tract rather than allowing
them to be absorbed.
Where could you find this information? I found it on the web by doing a
simple search for the word "Olestra", but I have also seen the
structure in popular newsmagazines.
2. Sickle cell anemia is a genetic defect in the structure of hemoglobin,
in which just one amino acid is altered. One glutamic acid is replaced by
a valine. Describe why this change could be so serious for the protein tertiary
structure. Suggest a possible code in the DNA that might have been altered
in order to read Val instead of Glu.
The Glu residue in hemoglobin carries a negative charge and may be important
for interacting with a positive charge somewhere or just for making that
part of the molecule water-soluble. A change to Val makes that residue nonpolar.
It may be that the Val residue avoids water or the other polar environment
where Glu was normally found, and this causes the drastic conformational
change in tertiary structure that leads to sickle-shaped red cells.
The m-RNA codons for Glu are GAA and GAG.
The m-RNA codons for Val are GUX (where X can be any of the four bases).
The most likely error is that a GUA or GUG was accidentally read instead
of GAA or GAG. In both cases, U appears in the second base where A should
have been.
