Chemistry 332 - Spring 1996
Elements of Organic Chemistry II

Homework, Chapter 11 - Condensation Reactions
McMurry, pp 346-349:
Problems 11.18, 20, 22, 23, 26, 27, 29, 32, 35, 36, 46
1. Condensation reactions seem to have developed more "name"
reactions than most other areas of organic chemistry. For each reaction
below, identify the alpha-nucleophile and the carbonyl group that would
have been involved.
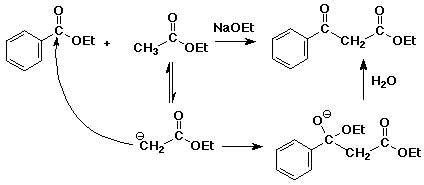
a) Perkin condensation
The alpha-nucleophile comes from the acid anhydride and condenses with the
aldehyde to give a beta-hydroxy acid anhydride. The reaction is completed
by hydrolysis of the anhydride and dehydration of the alcohol.
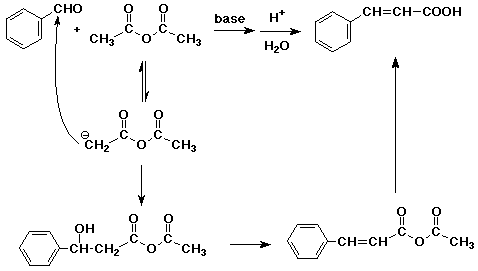
b) Knoevenagel condensation
The alpha-nucleophile comes from the diester (malonic ester) and condenses
with the aldehyde to give a beta-hydroxy acid anhydride. The reaction is
completed by dehydration of the alcohol.
c) Dieckmann condensation
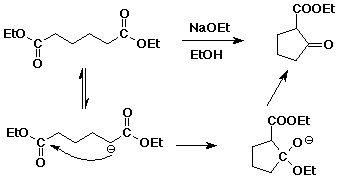
The alpha-nucleophile is adjacent to one of the two ester groups, and then
attacks the other ester, to give a beta-keto ester, which in this case is
cyclic.
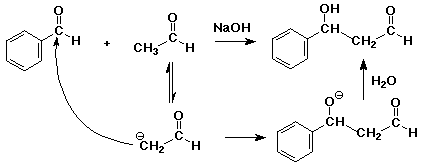
2. A crossed-aldol reaction simply implies that two different components
were used, one to act as the nucleophile (electron donor) and one as the
carbonyl (electron acceptor). Write a complete mechanism for the crossed
aldol reaction between acetaldehyde and benzaldehyde. Indicate which you
selected to be the donor and which the acceptor, and also indicate how you
might arrange the experiment so that you avoid or minimize the extent of
normal aldol reaction.
3. Write a complete mechanism for a crossed Claisen condensation between
ethyl acetate and ethyl benzoate, using sodium ethoxide as the base.