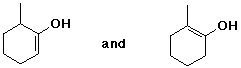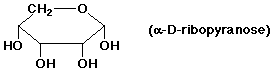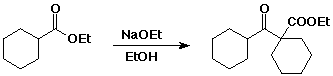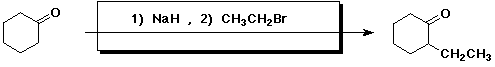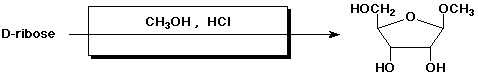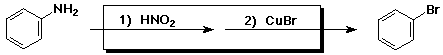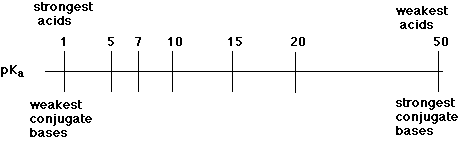Chemistry 332 - Spring 1996
Elements of Organic Chemistry II

Professor Carl C. Wamser

EXAM 2 - Answer Key
May 16, 1996
1. (15 points) Write a complete name for each of the following compounds:
a) 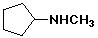
cyclopentylmethylamine
or methylaminocyclopentane
or N-methylcyclopentanamine
b) 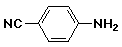
p-cyanoaniline
or 4-cyanoaniline
or p-aminobenzonitrile
or 4-aminobenzonitrile
c) 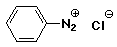
benzenediazonium chloride
d) 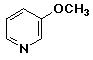
3-methoxypyridine
or m-methoxypyridine
e) 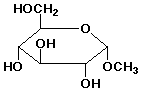
methyl alpha-D-glucopyranoside
2. (15 points) Write accurate structures for each of the following:
a) enol tautomers of 2-methylcyclohexanone
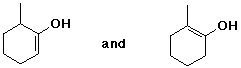
b) an aldopentose in a pyranose ring
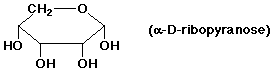
c) a chiral quaternary ammonium ion

d) the Claisen condensation product from ethyl cyclohexanecarboxylate
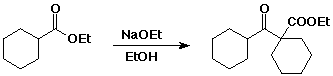
e) an alpha-1,6'-glycoside linkage between two D-glucose units
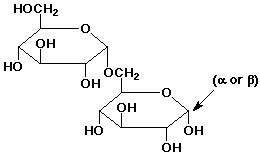
3. (15 points) Complete each of the following reactions by adding the
missing part:
a) 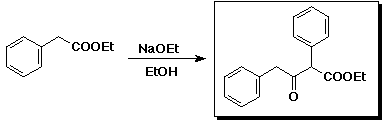
b) 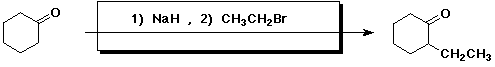
c) 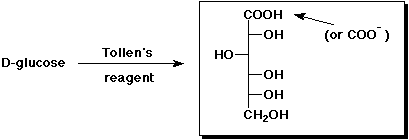
d) 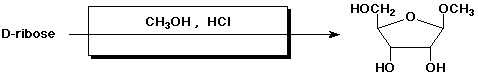
e) 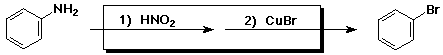
4. (15 points) Write a complete mechanism for the aldol reaction of
acetone, catalyzed by NaOH, including the dehydration of the aldol product.

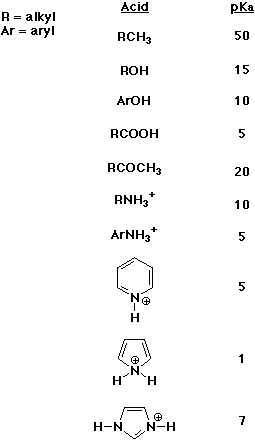
5. (10 points) Identify the approximate pKa values of the ten functional
groups or families shown below. Select from only the seven possible pKa
values shown on the line below.
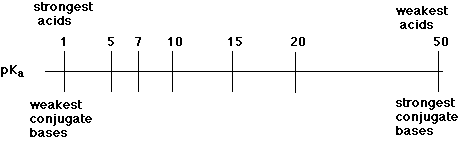
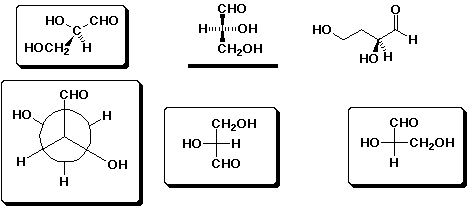
6. (10 points) Examine the structures shown below. Circle those that
represent D-glyceraldehyde and underline those that represent L-glyceraldehyde.
7. (10 points) L-Rhamnose, shown below, is one of the few naturally
occurring L-sugars. Note that it is also a 6-deoxy aldohexose.
Write a Haworth projection and the best chair form for alpha-L-rhamnopyranose.

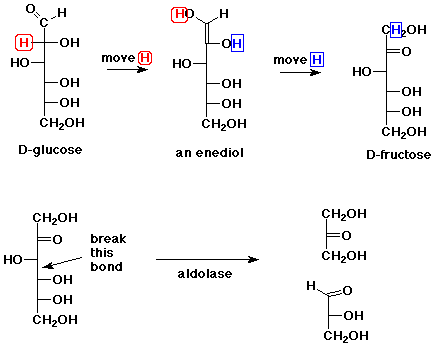
8. (10 points) In the metabolic breakdown of sugars, D-glucose is converted
into D-fructose via an enol (actually an enediol in this case). D-Fructose
is then split into two trioses via a reverse aldol reaction (by the enzyme
aldolase). Show these two reactions, clearly indicating where new bonds
are made or broken.