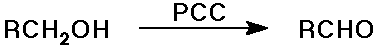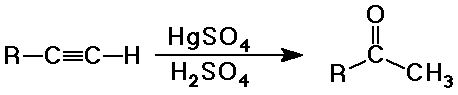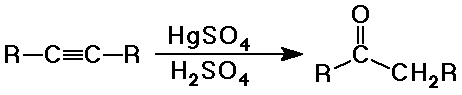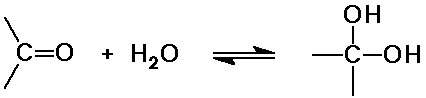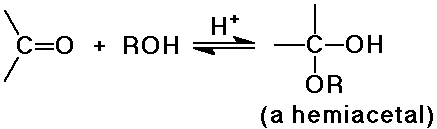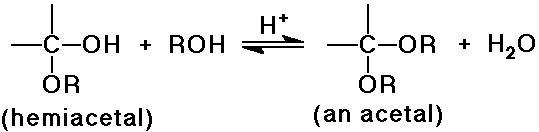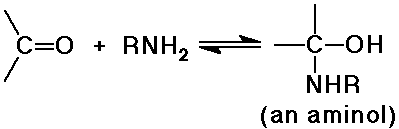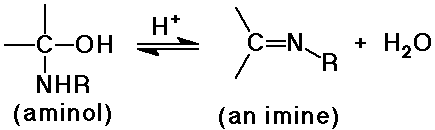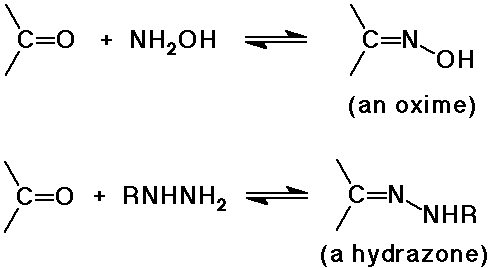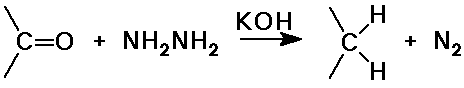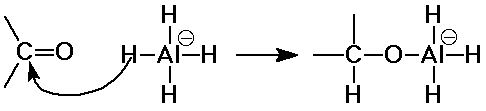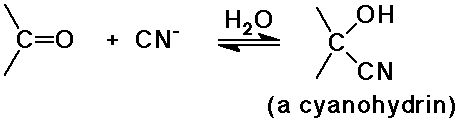Chemistry 332 - Spring 1996
Elements of Organic Chemistry II

Professor Carl C. Wamser

Chapter 9 - Carbonyl Compounds
Tues, April 9
Carbonyl Functional Groups
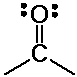
usually subdivided into two families:
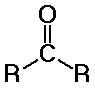 aldehydes and ketones
aldehydes and ketones
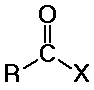 carboxyl derivatives
carboxyl derivatives
- X is some electronegative element
- (halogen, O, N, S, others)
- covered in Chapter 10
The Carbonyl Group - Structure and Properties
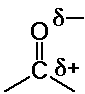
- polar C=O double bond
- O is nucleophilic
reacts with acids & electrophiles
- C is electrophilic
reacts with Lewis bases and nucleophiles
Aldehyde Nomenclature
-al suffix, with carbonyl assumed #1 in parent chain
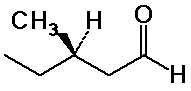 (R)-3-methylpentanal
(R)-3-methylpentanal
-carbaldehyde suffix
(if aldehyde can't be part of the parent)
e.g., cyclohexanecarbaldehyde
formaldehyde
acetaldehyde
propionaldehyde
butyraldehyde
benzaldehyde
Ketone Nomenclature
-one suffix, with number
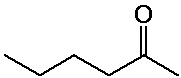 2-hexanone
2-hexanone
-oxo- prefix if necessary
(when it must be a substituent within the parent,
because another functional group takes higher priority
- such as aldehyde)
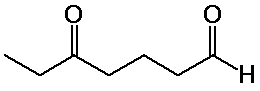 5-oxoheptanal
5-oxoheptanal
acyl- as prefix if necessary
(when the carbonyl isn't be part of the parent)
acetyl
benzoyl
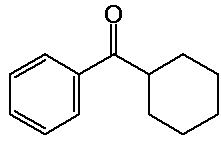 benzoylcyclohexane
benzoylcyclohexane
dialkyl ketone
acetone
acetophenone
benzophenone
Nomenclature examples:
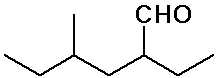 2-ethyl-4-methylhexanal
2-ethyl-4-methylhexanal
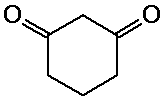 1,3-cyclohexanedione
1,3-cyclohexanedione
Aldehyde Syntheses
- oxidation of primary alcohols with PCC
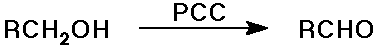
Ketone Syntheses
- oxidation of secondary alcohols
with KMnO4, CrO3, PCC, Na2Cr2O7

- hydration of terminal or symmetrical alkynes
(other alkynes give mixtures)
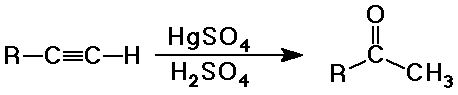
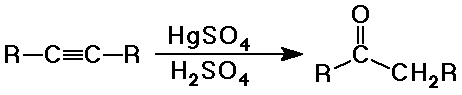
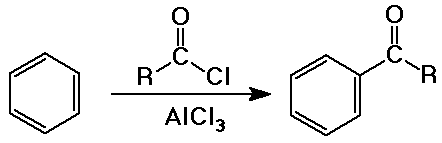
- Friedel-Crafts acylation of aromatics
Oxidation of Aldehydes
- very sensitive to oxidation
(slow reaction with air by a radical chain mechanism)
- Tollen's test
Ag+ in aqueous ammonia reduced to metallic silver (mirror)
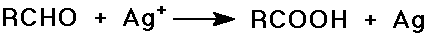
Nucleophilic Addition to Carbonyls
the major reaction of carbonyl compounds
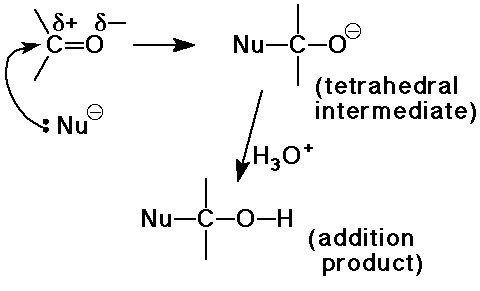
General Trends:
- aldehydes more reactive than ketones
(due to steric hindrance with ketones)
- aromatic carbonyls less reactive than aliphatic
(due to conjugation effects)
- a large variety of nucleophiles can undergo addition reactions
O nucleophiles: OH-, H2O, ROH
N nucleophiles: NH3, RNH2
H nucleophiles: LiAlH4, NaBH4
C nucleophiles: CN-, RLi, RMgX
Acid Catalysis of Nucleophilic Addition
- protonation generates a more reactive electrophile
(carbon becomes more receptive to nucleophiles)
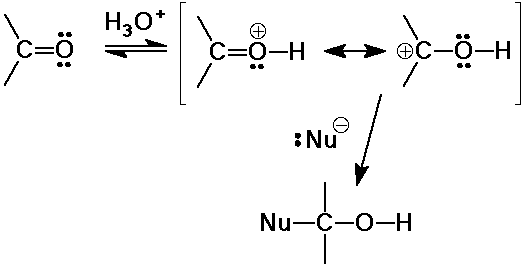
- works well to enhance reactivity with weak nucleophiles
- doesn't work for nucleophiles that protonate readily
Hydration Reactions
- as in hydration of C=C double bonds,
the elements of H and OH are added
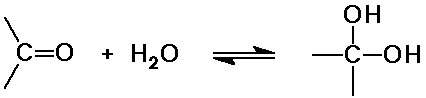
- H2O is the nucleophile
- the product is a geminal diol
Hydration is a reversible equilibrium
- formaldehyde in water is mainly dihydroxymethane
- most other carbonyls have unfavorable equilibria
(very little gem-diol in aqueous solution)
- reaction rate is slow at pH 7
but faster in acid or in base (catalysis)
Base-Catalyzed Hydration
- OH- is a strong nucleophile
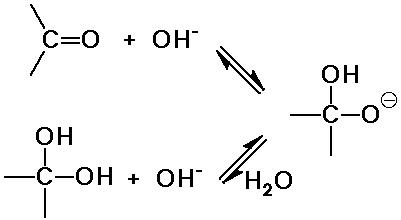
Acid-Catalyzed Hydration
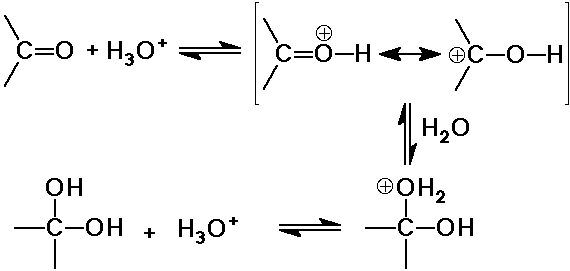
Comparison of acid and base catalytic effects
- in base, a strong nucleophile is generated by deprotonation
- in acid, the carbonyl is made more receptive to a weak nucleophile
- note that you can't have both at the same time
(although nature comes close with some enzyme catalysts)
Alcohol Additions
- reversible addition gives a hemiacetal, which is usually unstable
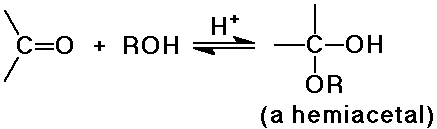
- the hemiacetal undergoes acid-catalyzed substitution (SN1)
to an acetal
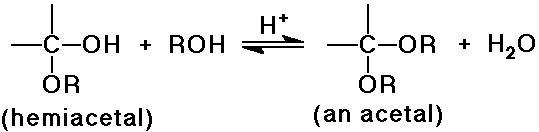
- ketal (and hemiketal) are terms often used for the corresponding products
from ketones
- reversible acid-catalyzed equilibrium
pushed forward by removal of water
reversed by addition of excess water (hydrolysis)
Acetals as protecting groups
- ether functional groups are mainly unreactive
- but easily removed by acid hydrolysis
- protecting groups temporarily "cover up" a reactive carbonyl
group
- usually to avoid unwanted reactions during other synthetic steps
Amine Additions
- reversible addition gives an aminol, which is usually unstable
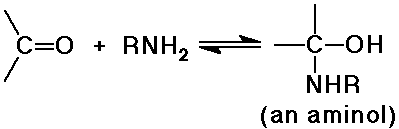
- the aminol dehydrates to an imine under acid catalysis
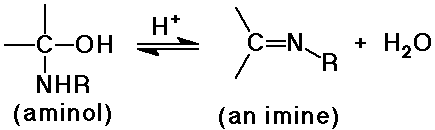
- acid-catalysis limited to mild pH ranges (typically 4 - 6)
- otherwise the amines are all protonated and no longer nucleophilic
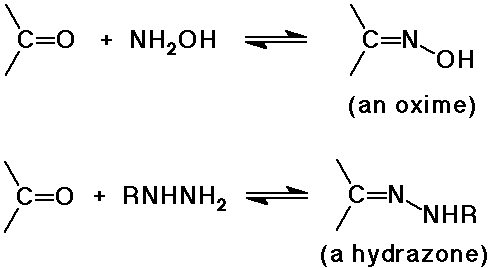
Wolff-Kishner reduction
- method for removal of a carbonyl group (to an alkane)
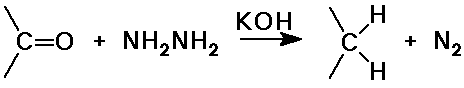
Hydride Additions
- reductions by LiAlH4 or NaBH4 are additions
of "H-"
- irreversible
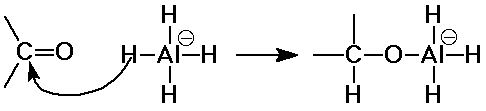
Carbon Additions
- cyanide additions gives cyanohydrins
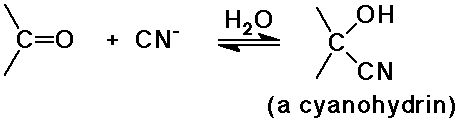
- organometallic reagents (nucleophilic carbon)
- add a new C-C bond and make alcohols
- addition is irreversible
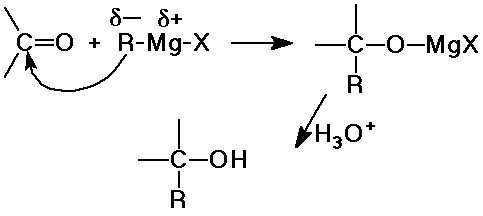
- Grignard + formaldehyde --> 1° alcohol
- Grignard + aldehyde --> 2° alcohol
- Grignard + ketone --> 3° alcohol
- useful synthetic reaction
a new C-C bond is formed
builds up a larger carbon skeleton
- Grignard reagents are incompatible with most other polar functional
groups
(OH, COOH, NH2, etc.) and with atmospheric water and oxygen
Natural Cyanohydrins
- some organisms use cyanohydrins to release toxic HCN as a defense
mechanism
Glucose Hemiacetals
- the major form of most sugars in aqueous solution is as cyclic hemiacetal
isomers
(covered in Chapter 14)
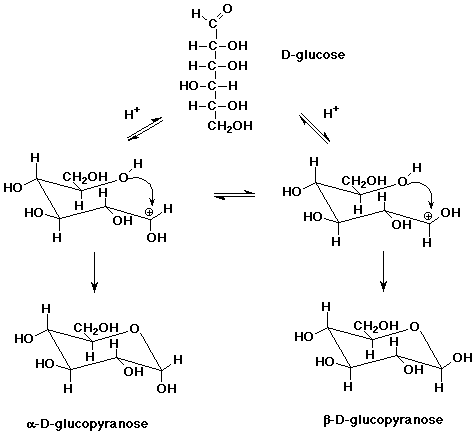
Glucose Acetal Polymers (Starches and Cellulose)
- the storage form of glucose in animals (starches)
and in plants (cellulose - structural material)
Skills from Chapter 9
- generate appropriate names for aldehydes and ketones
- predict the products of nucleophilic addition reactions
- write mechanisms for nucleophilic addition reactions
- recognize when acid or base catalysis is effective in nucleophilic
addition reactions
- use Grignard reactions in synthetic sequences
- use acetal protecting groups in synthetic sequences
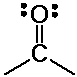
 aldehydes and ketones
aldehydes and ketones
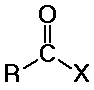 carboxyl derivatives
carboxyl derivatives

(R)-3-methylpentanal
2-hexanone
5-oxoheptanal
benzoylcyclohexane
 2-ethyl-4-methylhexanal
2-ethyl-4-methylhexanal 1,3-cyclohexanedione
1,3-cyclohexanedione