
Chemistry 332 - Spring 1996
Elements of Organic Chemistry II

Professor Carl C. Wamser

Chapter 16 - Lipids and Nucleic Acids
Thurs, May 23
Lipids
- general term for biomolecules that are water-insoluble but soluble
in organic media
(in biological systems this usually means membrane-bound)
- categories of lipids:
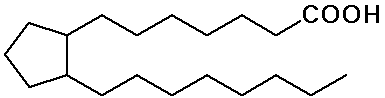
- fatty acids - long chain carboxylic acids (C12 - C20)
- fats and oils - triesters of fatty acids and glycerol (triglycerides)
(monoglycerides are monoesters, diglyecrides are diesters)
(function as energy storage materials)

- phosphatides - phosphate esters of glycerol (+ fatty acids)
(membrane constituents)

- steroids - polycyclic structure with specific substituents
(hormones, special functions)
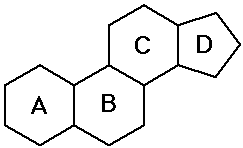
- prostaglandins - cyclic structure with long chains
(hormones, special functions)
Fats and Oils
- fats are solids, oils are liquids (same basic triglyceride structure)
- serve as insoluble deposits that can be retrieved for energy
Fatty Acids
- all natural fatty acids have an even number of carbon atoms, since
they are synthesized from acetate (C2) subunits
- natural unsaturated fatty acids have cis double bonds
cis-double bonds lead to a lower melting point
unsaturated and polyunsaturated fats are also more easily digestible
Soaps
- basic hydrolysis of fats (saponification) generates soaps
(the sodium salts of fatty acids)
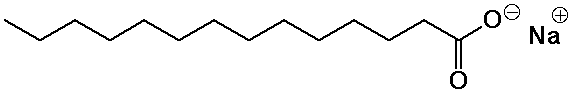
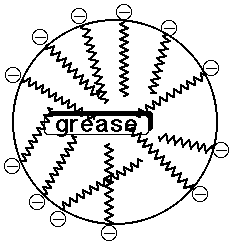
- the ionic head group is water-soluble, the nonpolar tail insoluble
- soaps tend to aggregate in micelles, where nonpolar dirt dissolves
Phospholipids
- the natural analog of the soap structure:
a polar phosphate head group
two nonpolar tails

Bilayer Lipid Membranes
- phospholipids with two tails tend to make planar bilayer aggregates
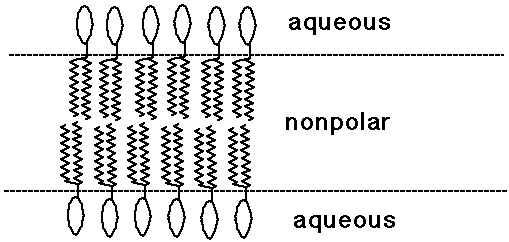
- other lipids are found in the nonpolar environment of membranes
- proteins often have a nonpolar and polar part, and are membrane-bound
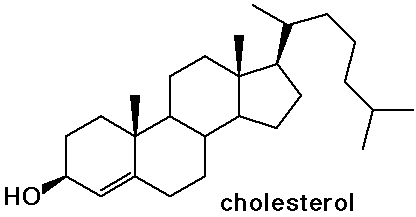
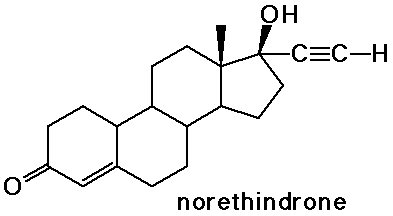
Steroids
- cholesterol is the precursor to most other steroids: vitamin D,
bile salts, hormones
- the sex hormones and birth control pills are steroids
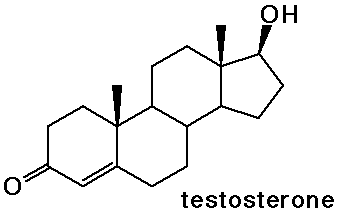
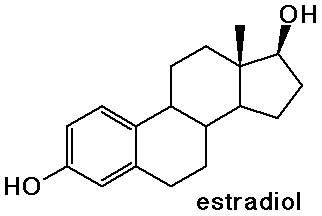
Nucleic Acids
- huge molecules that carry the genetic information for an organism
- a polymer composed of phosphate esters linking sugars, with specific
nitrogen bases attached to each sugar unit
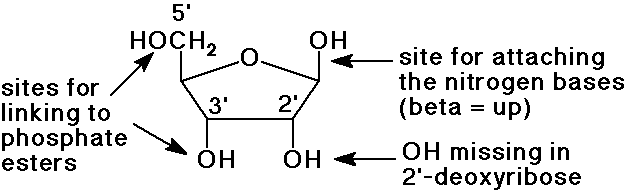
Deoxyribose
- in RNA, ribose is the sugar, specifically beta-D-ribofuranose
- in DNA, 2-deoxyribose is the sugar, also with the beta furanose
structure
The Nitrogen Bases
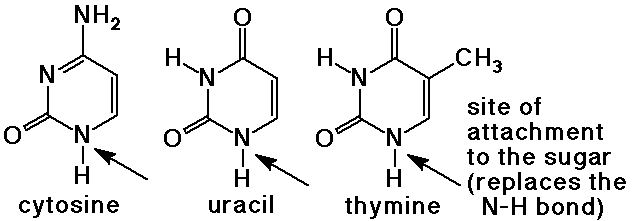
- pyrimidine bases have a 6-membered ring with two nitrogens
cytosine, uracil (found in RNA), and thymine (found in DNA)
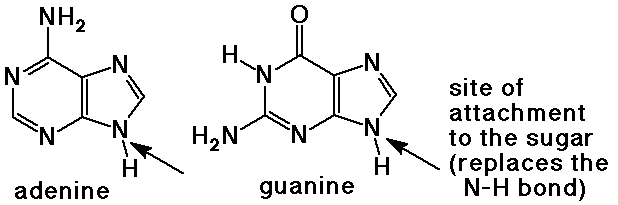
- purine bases have two rings with four nitrogen atoms
adenine and guanine
Nucleosides
- nitrogen base + sugar = nucleoside
- one-letter codes imply the nucleoside (add "d" for deoxy)
C = cytosine
U = uridine
T = thymidine
A = adenosine
G = guanosine
Nucleotides
- nitrogen base + sugar + phosphate(s) = nucleotide
- ATP = adenosine triphosphate is used as an energy storage molecule
hydrolysis to ADP releases 8 kcal/mole
coupling of ATP hydrolysis helps to drive reactions that would ordinarily
be unfavorable
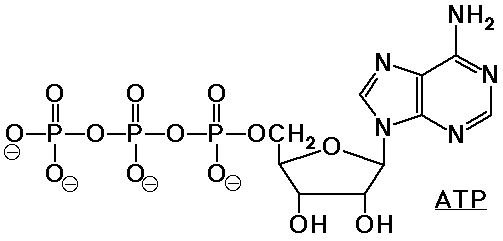
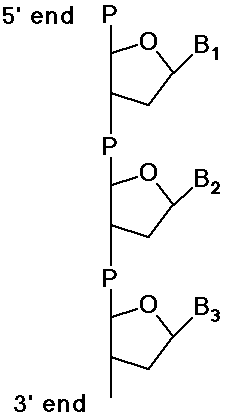
Polynucleotides - DNA and RNA
- polymers of DNA or RNA are named from 5' end to 3' end
Base Pairing
- specific H-bonding can take place between bases on adjacent strands
in DNA: A & T base pair, G & C base pair
in RNA: A & U base pair, G & C base pair
- the base pairing requires antiparallel directions of the two strands
the optimum structure for base pairing is a double helix
Replication of DNA
- note that each strand of DNA contains enough information to duplicate
its partner
- DNA is replicated by uncoiling and creating new partners for each
strand
- replication is done by an enzyme, DNA polymerase, using nucleotide
triphosphates (like ATP)
Transcription of Genetic Information from DNA to RNA
- information from DNA is transcribed by copying onto messenger RNA
(m-RNA)
- the m-RNA message matches the original DNA (complementary to the
DNA being copied)
Translation of the Genetic Information into Protein Biosynthesis
- m-RNA brings the genetic information to ribosomes, where protein
biosynthesis takes place
- m-RNA message is read sequentially by its 3-letter codons
- as needed, individual amino acids are brought to the ribosomes
by transfer RNA (t-RNA)
- t-RNA specifically recognizes both the code for an amino acid and
its particular amino acid
The Genetic Code
- each amino acid is represented by a three-letter code
the code is expressed as it appears on the m-RNA, from 5' to 3' direction
(Table 16.3 will be provided for quizzes and exams)
- special features of the genetic code (like a language)
- no punctuation - exact starting spot is crucial, must always
move by 3 units
- there is a "stop" code
- duplicate codes - most amino acids have multiple codes
- single-letter changes (especially in the last spot) usually don't
cause a serious misreading of the code
(however, sickle-cell anemia is caused by a single error in the structure
of hemoglobin, in which 6-Glu is erroneously replaced by 6-Val)
Why is the OH Missing in DNA?
- RNA (both m-RNA and t-RNA) is meant to recycle readily to read
and write messages
- DNA is meant to remain stable
- hydrolysis of the phosphate esters of nucleic acids is enhanced
by the 2'-OH group
in the absence of the 2'-OH group, the phosphate esters are very stable
to hydrolysis
DNA Sequencing
- sizes of DNA molecules measured in kilobases (1000s of base pairs)
- specific cleavages of DNA can occur with restriction enzymes
- smaller fragments are more easily identified and reassembled into
the original
- the Human Genome Project
Skills from Chapter 16
- draw structures for representative examples of fats, fatty acids,
unsaturated fats, soaps, phospholipids, steroids, nucleosides, nucleotides
- recognize how the structures are appropriate for their particular
biological functions
- identify the complementary base pairs in DNA and RNA
- identify the codons for amino acids and their sequence in DNA,
m-RNA, and t-RNA
- describe the processes of DNA replication, transcription, and translation
















