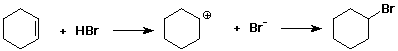Chemistry 331 - Winter 1996
Elements of Organic Chemistry I

Quiz, Chapter 3 - Alkenes
1. (3 points) Write a complete IUPAC name for each of the following
compound, including a designation of stereochemistry:
(Hint: It is more important to include a double bond in the parent
chain than a cycloalkane.)
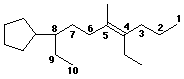
(E)-8-cyclopentyl-4-ethyl-5-methyl-4-decene
2. (2 points) Use the sequence rules to rank order the priority of the
groups listed below. Use 1 for the highest priority down to 5 for the lowest
priority.
1 -OCH3
2 -OH
3 -COOH
4 -cyclopentyl
5 -CH(CH3)2
3. (2 points) Identify the electrophile and the nucleophile in each of
the two reactions shown below:
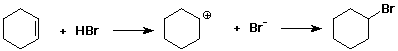
nucleophile + electrophile electrophile + nucleophile
4. (3 points) A potential energy diagram is shown for the reaction below.
CH4 + Cl -----> CH3 + H-Cl
delta H = + 1 kcal/mole
Ea = 4 kcal/mole
On the diagram, specifically locate the reactants, the products, Ea, H,
and the transition state.
*******
we'll do this in class, or get the picture from the library reserve folder
*******
Is this reaction exothermic or endothermic?
endothermic (uphill)
Draw what could be a logical structure for the transition state for this
reaction.
(Hint: Both Cl and CH3 are radicals, having only 7 valence electrons.
Don't let that bother you.)
CH3.....H.....Cl