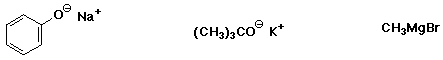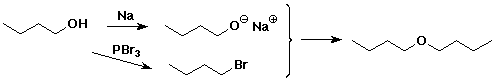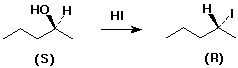Chemistry 331 - Winter 1996
Elements of Organic Chemistry I

Professor Carl C. Wamser

EXAM 3 - Answer Key
March 14, 1996
1. (15 points) Write complete names for each of the following, including
designation of stereochemistry if it is specifically shown.
a) 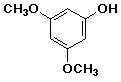
3,5-dimethoxyphenol
b) 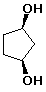
cis-1,3-cyclopentanediol
c) 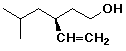
(S)-3-isobutyl-4-penten-1-ol
2. (15 points) For each of the following groups of compounds, arrange
them in order with respect to the particular property indicated.
Write "MOST" and "LEAST" under the most reactive and
least reactive in each series.
a) SN2 reactivity towards NaCN:

MOST , MIDDLE , LEAST
b) SN1 reactivity in neutral methanol solution:
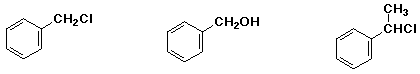
MIDDLE , LEAST , MOST
c) reactivity as an acid:
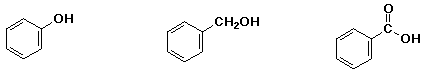
MIDDLE , LEAST , MOST
d) reactivity as an acid:
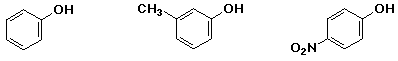
MIDDLE , LEAST , MOST
e) reactivity as a base:
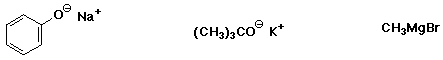
LEAST , MIDDLE , MOST
3. (15 points) Complete each of the following reactions by adding
the missing part: either the starting material, the necessary reagents and
conditions, or the expected major product. Indicate stereochemistry if it
would be specific.
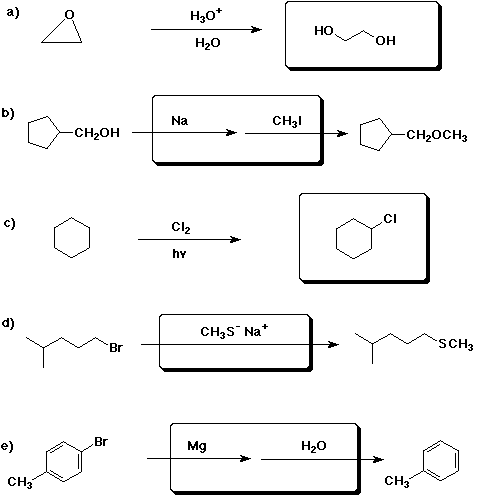
4. (10 points) Write a complete mechanism for the acid-catalyzed dehydration
of tert-butyl alcohol.

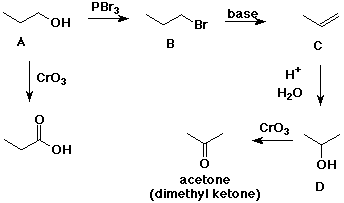
5. (15 points) Identify each of the lettered compounds in the scheme
below. Show all of the reactions that are referred to.
A is an alcohol that can be oxidized by CrO3 to give a carboxylic
acid.
When A is treated with PBr3, it gives compound B.
When B is treated with strong base, it gives compound C.
When C is hydrated using dilute sulfuric acid in water, it gives D, which
is an isomer of A.
Oxidation of D with CrO3 gives acetone (dimethyl ketone).
A must be a primary alcohol (RCH2OH) in order to be oxidized
to a carboxylic acid.
D must be 2-propanol, since that is the only alcohol that could be oxidized
to give acetone.
A must be 1-propanol.
6. (10 points) Develop a sequence of reactions that could be used to
synthesize one of the following compounds below. As starting materials,
you may use any alcohol, but no other organic compounds.
SELECT JUST ONE.
dibutyl ether OR (R)-2-iodopentane
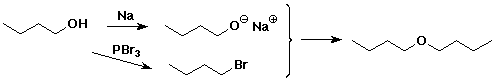
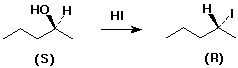
7. (20 points) 2-Bromobutane undergoes E2 elimination when it is treated
with KOH in ethanol as solvent. Three products are obtained:
1-butene, cis-2-butene, and trans-2-butene.
Predict the relative order of abundance of these three products (most, less,
least).
trans-2-butene (MOST) > cis-2-butene (LESS) > 1-butene (LEAST)
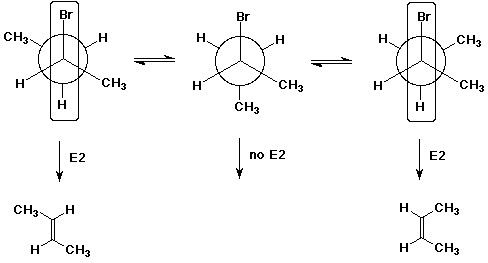
Write a Newman diagram for (R)-2-bromobutane, looking down the C2-C3
bond.
Show all three staggered conformations, and show why E2 elimination from
specific conformations may lead to specific products.
E2 elimination requires the H and the Br that are eliminated to be aligned
anti to one another. This may occur either while the two methyl groups are
also anti (leading to trans-2-butene) or while the two methyl groups are
gauche (leading to cis-2-butene).
Trans is the major product because it comes from the more stable conformation.
Write a Newman diagram looking down the C1-C2
bond, and illustrate how E2 elimination would give rise to 1-butene.
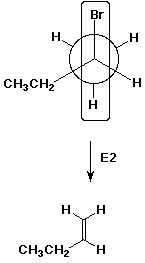
1-Butene would be a lesser product because it is less stable than the
more substituted 2-butenes.
Explain what would be different about your diagrams and explanations
if you were working with (S)-2-bromobutane instead of (R).
All the Newman diagrams would be the mirror images of the ones shown.
However, the same products would be formed from the enantiomeric structures
and in the same amounts.
![]()
![]()
![]()