![]()
![]()
![]()
1. (2 points each) Complete each of the following acid-base reactions. In each case, identify the acid and base on each side of the equation.
a) 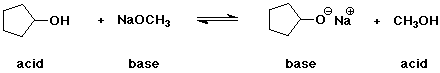
b) ![]()
2. (2 points each) Use the table of pKa values to predict whether the following reactions would be favored in the forward or reverse direction.
a) ![]()
Forward (CH3CO2H is a stronger acid than NH4+)
b) ![]()
Forward (CH3CH2OH is a stronger acid than NH3)
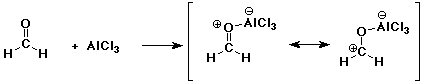
3. (2 points) Write a Lewis acid-base reaction involving the two compounds shown below. Include resonance forms for the product.