![]()
![]()
![]()
1. (4 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product.
a) 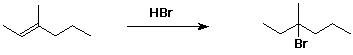
b) 
c) 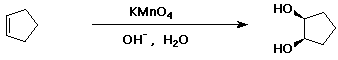
d) 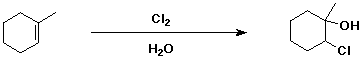
2. (3 points) Write a complete mechanism for the addition of HCl to 1-methylcyclobutene. Show all steps and all intermediate structures, including electron-pushing arrows.
3. (3 points) The above reaction has two steps with H1 = + 20 kcal/mole, H2 = - 28 kcal/mole, and Htotal = - 8 kcal/mole.
Draw an approximate potential energy reaction diagram, labeling all the important components, including the axes, each of the H values given, Ea, and the location of the reactants, products and intermediates.
Identify which step you predict would be the rate-determining step.
The first step is highly endothermic and is most likely to be the rate-determining step.