![]()
![]()
![]()
1. (4 points) Write the best Lewis structure, including all lone pairs,
for each of the two constitutional isomers that would be represented in
condensed formulae as
CH3COOH and HCOOCH3.

2. (2 point) Identify the hybridization of each carbon in the structures above.
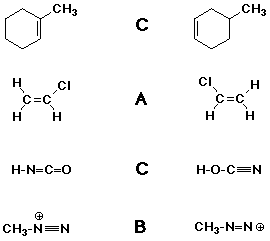
3. (4 points) Identify the relationship between the molecules in each
pair below.
Possibilities are:
A - they are the same molecule
B - they are resonance forms of the same molecule
C - they are constitutional isomers