![]()
![]()
![]()
(1 point each)
1. From its name, you can tell that hexane has _____6______ carbons.
Similarly, the alkane with eight carbons is called _____octane_________ .
2. On the other hand, you need to remember all the special names for the smaller alkanes:
Structure Name
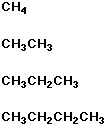 methane, ethane, propane, butane
methane, ethane, propane, butane
3. In alkanes, all of the bonds are ( polar / nonpolar ) , so alkanes
typically ( do / do not ) dissolve in water. (Circle one from each pair)
4. IUPAC is the acronym for the organization that, among other things, developed a comprehensive system for . . . naming organic compounds.
5. When alkanes burn, all carbon atoms oxidize to _____CO2________ ,
and all hydrogen atoms oxidize to ____H2O_________ .