![]()
![]()
![]()
Brown, pp 271 - 276:
Problems 9.8 - 11 , 13 - 14 , 17 - 28 , 32 - 33 , 35 - 37 , 39
1. Write complete names for each of the following compounds:
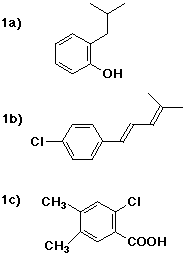
1a) o-isobutylphenol
1b) (1E)-1-(p-chlorophenyl)-4-methyl-1,3-pentadiene
1c) 2-chloro-4,5-dimethylbenzoic acid
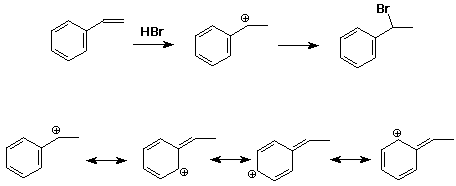
2. Apply the Markovnikov Rule to the addition of HBr to styrene (phenylethene). For the predicted intermediate cation, write all the resonance forms.
The electrophile (H+) will add to the end of the pi bond and form a benzylic cation that is stabilized by resonance in a manner similar to an allylic cation.
3. Consider whether the nitroso group (-N=O) would be activating or deactivating, and whether it would be ortho,para or meta directing. Justify your choices.
It is an activating o,p-director.
The lone pair on N can be donated to the intermediate cation to stabilize the positive charge by resonance.
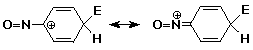
A case could also be made for -N=O as electron-withdrawing since O is more electronegative than N and the N=O bond is polarized with N partially positive.