![]()
![]()
![]()
Brown, pp 200 - 205 :
Problems 7.12 - 18 , 21 , 24 - 27 , 31 - 37 , 39 - 44 , 47
1. Give complete names for each of the following:
a)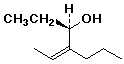
(R),(Z)-4-propyl-4-hexen-3-ol
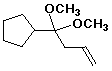
4-cyclopentyl-4,4-dimethoxy-1-butene
2. Write a complete mechanism for the addition of a methyl Grignard reagent
(CH3MgBr, a strong nucleophile) to 1,2-epoxycyclohexane. Explain why this
might be called an SN2 mechanism.

There is a backside attack on a carbon by a nucleophile and a leaving
group (O) that is displaced. It works here and not for most ethers because
the ring strain is relieved when the leaving group is displaced.
3. Tert-butyl ethers are sometimes used as protecting groups because they
can be easily put on and easily removed. Write a complete mechanism for
the cleavage of t-butyl pentyl ether by HBr.

4. Consider the four alcohols of formula C4H10O. Isomer A does not react
with CrO3. Isomers A and B do not react with PCC. Isomer C is formed from
reduction of butanoic acid (CH3CH2CH2COOH) with LiAlH4. Identify the four
isomers by IUPAC name, common name, and structure. Write the names and structures
of all other isomers that would have the formula C4H10O.
A = t-butyl alcohol (2-methyl-2-propanol)
B = sec-butyl alcohol (2-butanol)
C = butyl alcohol (1-butanol)
D = isobutyl alcohol (2-methyl-1-propanol)
Other constitutional isomers are ethers:
diethyl ether
methyl propyl ether
methyl isopropyl ether