![]()
![]()
![]()
Brown, pp 119 - 123 :
Problems 4.6 - 16, 18, 22 - 27, 29, 30, 32 - 37
1. When chlorine is added to an alkene in aqueous (water) solution, another major product besides the dichloro addition product is usually observed. For example, propene reacts with aqueous chlorine to form 1-chloro-2-propanol as the major product.
Write a mechanism that explains how this product forms.
Indicate how formation of this product relates to the Markovnikov rule.
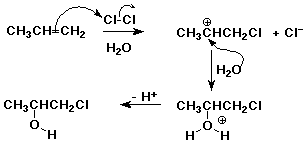
The electrophile was Cl, which added first so as to form the more stable carbocation. So Cl added to the less-substituted carbon and the nucleophile (OH) aded to the more substituted carbon. This is consistent with the Markovnikov Rule, considering electrophile and nucleophile in place of H and X.
2. Many alkenes undergo polymerization not only by free radical polymerization, but also by cationic polymerization. During World War 2, as a substitute for natural rubber, butyl rubber was made by cationic polymerization of isobutylene (2-methylpropene).
Write a chain reaction mechanism for cationic polymerization of isobutylene, using strong acid (H+) as initiator.
Explain why isobutylene would be particularly favorable for this type of reaction.
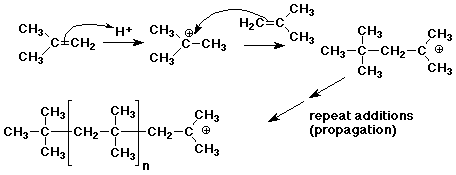
In each case of chain growth, a 3° carbocation is formed, which is relatively stable.