![]()
![]()
![]()
1. (15 points) Write a complete IUPAC name for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) 
b) 
c) 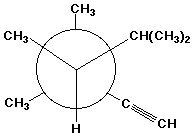
2. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Indicate stereochemistry if it is specific.
a) ![]()
b) ![]()
c) ![]()
d) 
e) 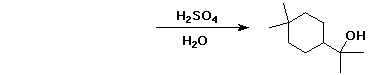
3. (10 points) The structure of D-fructose is shown below in a Fischer projection.
How many stereocenters are in this molecule ?
How many stereoisomers of D-fructose are there ?
How many of these stereoisomers are meso compounds ?
How many of these stereoisomers are diastereomers of D-fructose ?
What is the relationship of D-fructose to the other stereoisomers (the ones that aren't diasteromers) ?
4. (15 points) The pKa of CH3OH2+ is - 2.2 .
Write out the specific reaction that this refers to.
Write the Ka in terms of concentrations.
Knowing this pKa value, predict the preferred direction of the following equilibria:
5. (15 points) Write all the steps in the complete mechanism for the addition of HBr to 1-methylcyclohexene. Show electron-pushing arrows. Predict which is likely to be the rate-determining step.
6. (15 points) Addition of HCl to 3-methyl-1-butene gives the expected Markovnikov product, but also gives some 2-chloro-2-methylbutane.
Explain by writing a complete mechanism that shows how both products could be formed.
7. (15 points) Hydroxide ion could react with acetaldehyde (shown below) in two different ways.
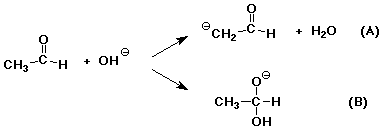
Clearly show electron-pushing arrows to illustrate what happens in each reaction.
Describe each reaction type (i.e. , what has happened?)
(A) -
(B) -
Assume both reactions are favorable, and B is more favorable than A, yet A is a faster reaction than B. Draw an approximate potential energy diagram that would be consistent with this.