![]()
![]()
![]()
1. (15 points) Write a complete IUPAC name for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) 
(R)-3-chloro-3-methylcyclopentene
b) 
(4E,7S)-7-bromo-2,4,6-trimethylocta-2,4-diene
c) 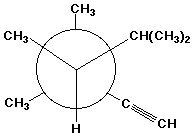
(R)-3,3,4,5-tetramethyl-1-hexyne
2. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Indicate stereochemistry if it is specific.
a) 
b) 
c) 
d) 
e) 
(the second isomer may be more likely, since the first isomer would be expected to give a mix of two tertiary alcohols as products)
3. (10 points) The structure of D-fructose is shown below in a Fischer projection.
How many stereocenters are in this molecule ?
3
How many stereoisomers of D-fructose are there ?
8 (including D-fructose)
How many of these stereoisomers are meso compounds ?
none
How many of these stereoisomers are diastereomers of D-fructose ?
6
What is the relationship of D-fructose to the other stereoisomers (the ones that aren't diasteromers) ?
one is D-fructose itself and one is its enantiomer
4. (15 points) The pKa of CH3OH2+ is - 2.2 .
Write out the specific reaction that this refers to.
Write the Ka in terms of concentrations.
Knowing this pKa value, predict the preferred direction of the following equilibria:
Forward, since HCl is a stronger acid than CH3OH2+ .
Backward, since CH3OH2+ is a stronger acid than H3O+ .
5. (15 points) Write all the steps in the complete mechanism for the addition of HBr to 1-methylcyclohexene. Show electron-pushing arrows. Predict which is likely to be the rate-determining step.

The first step is most likely rate-determining, since it requires breaking two bonds and only making one. The second step is only one new bond making.
6. (15 points) Addition of HCl to 3-methyl-1-butene gives the expected Markovnikov product, but also gives some 2-chloro-2-methylbutane.
Explain by writing a complete mechanism that shows how both products could be formed.
Following Markovnikov's Rule, a 2° carbocation is initially formed. However, there is an adjacent 3° carbon, and the cation can be shifted there if a hydrogen atom (with 2 electrons) is shifted over to the 2° carbon. This is favorable since 3° carbocations are more stable than 2°.
The H shift will be more apparent if you don't use line structures (i.e., write out all atoms and bonds).
7. (15 points) Hydroxide ion could react with acetaldehyde (shown below) in two different ways.

Clearly show electron-pushing arrows to illustrate what happens in each reaction.
Describe each reaction type (i.e. , what has happened?)
(A) - acid-base reaction
(B) - addition reaction
Assume both reactions are favorable, and B is more favorable than A, yet A is a faster reaction than B. Draw an approximate potential energy diagram that would be consistent with this. Your diagram should show both reactions starting from the same place.
Reaction B is more exothermic than reaction A, yet A must have a lower energy of activation than B in order to be faster. Note that this necessarily requires that the potential energy diagrams must cross.