![]()
![]()
![]()
1. (15 points) Write a complete IUPAC name for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) 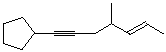
(E)-1-cyclopentyl-4-methylhept-5-en-1-yne
(trans is also OK)
b) 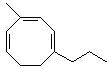
4-methyl-1-propylcycloocta-1,3,5-triene
c) 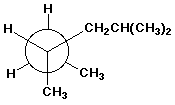
2,4-dimethylhexane
2. (10 points) Examine the pairs of structures below and identify their relationship to one another, using the letter codes below:
A - identical structures
B - conformational isomers
C - resonance forms
D - constitutional isomers
E - cis-trans isomers
F - none of the above
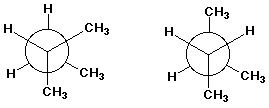 B
B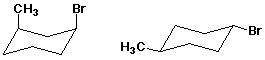 D
D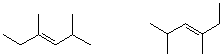 A or B
A or B
3. (10 points) Write complete Lewis structures for the three isomers shown below. Show all bonds, all lone pairs, and any formal charges.
One of the three has an alternative resonance form of about equal energy. Show both forms.
HSCN , HNCS , HCNS
4. a) (10 points) Identify the hybridization of each carbon in the compound below.
b) (10 points) Identify the number of hydrogens of each classification in the compound shown below.
primary (1°) - 11
secondary (2°) - 8
tertiary (3°) - 2
quaternary (4°) - 0 (there is a 4° carbon, but no hydrogens)
other - 1
The alcohol functional group would be described as ____ primary ____ .
5. (8 points) Complete the structures below by adding the missing parts. In each case, the structure on the left is complete and the structure on the right is the same structure from a different view, but missing some substituents.
a) 
b) 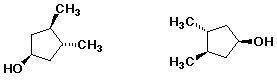
c) 
d) 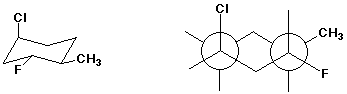
6. (10 points) Identify the following alkenes as either E or Z. No other naming is necessary.
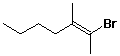 E
E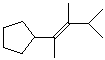 E
E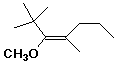 E
E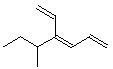 Z
Z7. (15 points) Write names for all of the possible isomers that would have the name dimethylcyclohexane. Consider mirror-image isomers as one compound. Structures are not necessary (unless it helps you find all the isomers).
1,1-dimethylcyclohexane
cis-1,2-dimethylcyclohexane
trans-1,2-dimethylcyclohexane
cis-1,3-dimethylcyclohexane
trans-1,3-dimethylcyclohexane
cis-1,4-dimethylcyclohexane
trans-1,4-dimethylcyclohexane
Identify which compounds would exist as two chair conformations of equal energy.
1,1-dimethylcyclohexane
cis-1,2-dimethylcyclohexane
trans-1,3-dimethylcyclohexane
cis-1,4-dimethylcyclohexane
Select one isomer that has two chair conformations of unequal energy and write the structure for the better conformation.
trans-1,4-dimethylcyclohexane
8. (12 points) The origin of steroids in many organisms is from cyclization of squalene into lanosterol, as shown below.
Indicate the isoprene units in squalene, include a designation of heads and tails.
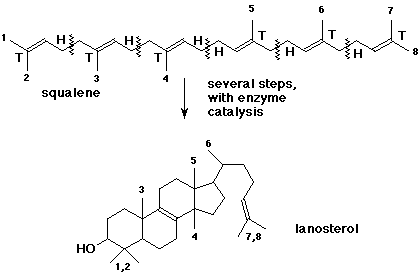
Lanosterol has 8 methyl groups. Indicate their origins from squalene by numbering the 8 methyl groups in both compounds.
Give the molecular formula for lanosterol.
C30 H50 O