![]()
![]()
![]()
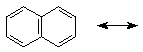
one resonance form of naphthalene is shown below - write all the others that involve simply shifting double and single bonds (i.e., no charges, all carbons with four bonds)
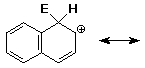
electrophilic aromatic substitution on naphthalene gives the carbocation
intermediate shown below - write all other resonance forms
write a structure for the resonance hybrid that shows the positions where
the positive charge is expected
any reaction that could generate a carbocation could initiate a Friedel-Crafts
substitution reaction
shown below is an industrial synthesis that makes cumene from benzene and
propene
note that there are no side products and H2SO4 is a catalyst (recycles)
- these are important considerations in an economic industrial process
write a complete mechanism, including all resonance forms for the intermediate
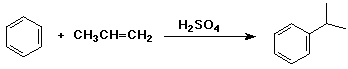
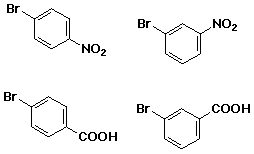
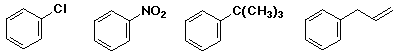
write the reactions you would use to make each of the following from
benzene:
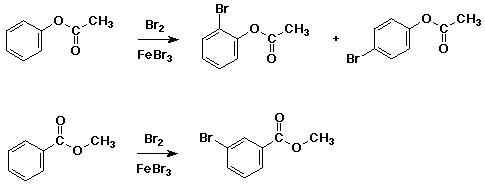
both substituents shown below are ester functional groups, yet one acts as a meta-director and one as an ortho, para-director - explain
which of the reactions above would you predict to be faster?
starting from benzene, develop synthetic sequences for each of the
following: