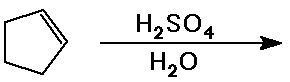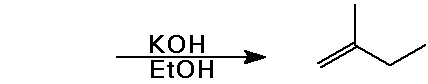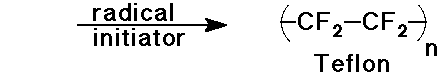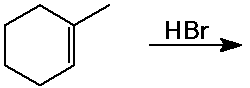![]()
![]()
![]()
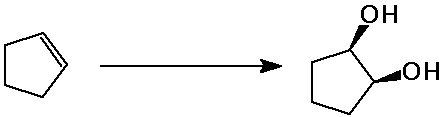
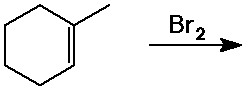
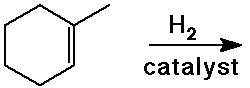
Predict the product expected from the following addition reactions:
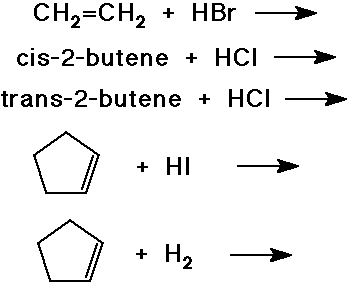
Draw electron arrows that indicate the flow of electrons in each reaction
below.
Also indicate electrophiles and nucleophiles in each reaction.
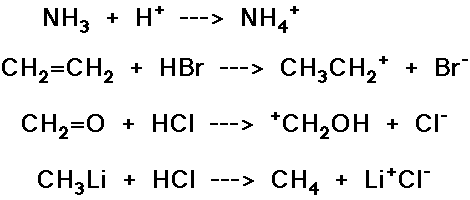
The isomerization of cis-2-butene to trans-2-butene can be accomplished by heating, with
delta H = - 5 kcal/mole
Ea = 55 kcal/mole
Draw an approximate potential energy diagram for this reaction.
Draw an approximate potential energy diagram for the reverse reaction
(i.e., the isomerization of trans-2-butene to cis-2-butene).
What would be delta H and Ea ?