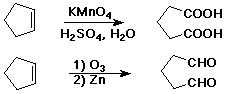Portland State University - - Professor Carl C. Wamser

Chapter 4 Notes - Alkene Reactions

Reaction Types
- additions
A + B --> C
H-Cl + CH2=CH2 --> CH3-CH2-Cl
- eliminations
X --> Y + Z
CH3-CH2-Cl --> CH2=CH2 + H-Cl
- substitutions
A-B + C-D --> A-C + B-D
CH4 + Cl2 --> CH3-Cl + H-Cl
- rearrangements
X --> Y
cyclopropane --> propene
Reaction Mechanisms
- a step-by-step account of how a reaction occurs (and why)
- bond-breaking steps:
homolytic: one electron to each fragment
(generates radicals)
heterolytic: both electrons to one fragment
(generates ions)
- bond-making steps:
homogenic or heterogenic
Electron Movement
- electron-pushing arrows indicate the flow of electrons in a mechanism
- electrophile:
electron-deficient species seeking a pair of electrons (a Lewis acid )
- nucleophile:
electron-rich species that can provide a pair of electrons (a Lewis base)
HCl plus Ethene
- CH2=CH2 + H-Cl --> CH3-CH2-Cl
- an electrophilic addition
- reaction type: addition
- reagent type: an electrophile
HCl, actually H+, a strong Lewis acid
Addition Mechanism
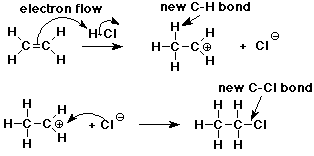
- pi bond is relatively reactive, especially towards electrophiles
it provides a good source of electrons
- addition of H+ to CH2=CH2 forms a new C-H sigma bond
the electrons for the new bond came from the pi bond
the other C is left with only 6 e-
Carbocation Intermediate
- an intermediate is formed in the reaction mechanism
CH2=CH2 + H+ --> CH3-CH2+
- carbocation: a carbon atom with only 3 bonds (6 e-) and a positive
charge
structure: sp2 hybridized (trigonal)
Formation of Chloroethane
- the reaction is completed as chloride anion (a nucleophile) adds to
the carbocation (an electrophile)
CH3-CH2+ + Cl- ---> CH3-CH2-Cl
Equilibrium
- for a general reaction:
A + B <==> C + D
- equilibrium constant, K
K = [C] [D] / [A] [B]
- favorable reactions have large K
- unfavorable reactions have small K
Kinetics
- rates of reaction
- may or may not correlate with the favorability of the equilibrium
- rate depends on the mechanism
- is there a good way to get from reactants to products?
Heats of Reaction
- delta H (enthalpy change)
delta H = H(products) - H(reactants)
- exothermic means delta H < 0 (negative)
reaction gives off heat
- endothermic means delta H > 0 (positive)
reaction absorbs heat
Potential Energy Diagrams
- draw exothermic reactions downhill
- draw endothermic reactions uphill
Activation Energy, Ea
- there is usually an energy barrier between reactants and products
- activation energy represents the highest amount of energy necessary
while travelling along the minimum-energy (easiest) pathway from reactants
to products
The Transition State
- structure of the molecule(s) at the highest point along the reaction
pathway
- the stability of the transition state (relative to reactants) determines
Ea (rate of reaction)
Alkene Addition Reactions
- pi bonds undergo addition reactions
CH2=CH2 + HCl --> CH3CH2Cl
- in general,
C=C + HX --> H-C-C-X
- alkenes react with hydrogen halides to form alkyl halides
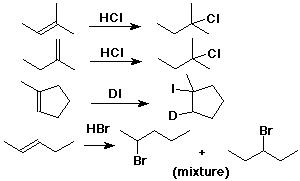
Addition of HX to Alkenes
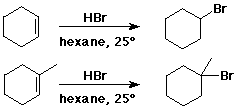
- cyclohexene + HBr --> bromocyclohexane
- 1-methylcyclohexene + HBr --> 1-bromo-1-methylcyclohexane
(not 1-bromo-2-methylcyclohexane)
Reaction Notation
- reactants -------> products
focus on the organic reactants and products
- show reagents over the arrow
- show solvent and conditions under the arrow
(or show full balanced reaction)
Orientation of Addition
- regiochemistry:|
specific orientation of addition
(which C gets H, which gets X?)
- alkene additions are regioselective:
one direction of addition is usually preferred
Markovnikov's Rule
- the original:
add H to the C with more H's
(or to the C with fewer alkyl groups)
- the reason:
add H+ to form the more stable cation
CH3CH=CH2 + HCl --->
CH3CH+CH3 (not CH3CH2CH2+)
---> CH3CHClCH3 (not CH3CH2CH2Cl)
Carbocations
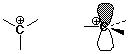
- structure: trigonal (sp2)
- stability: 3° > 2° > 1°
- more alkyl groups stabilize a cation by electron donation to the electron-deficient
(6-electron) carbocation
Markovnikov Addition
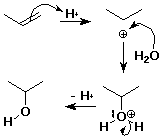
Hydration of Alkenes
- alkene + water --> alcohol
CH2=CH2 + H2O --(H+)--> CH3CH2OH
- mechanism:
- step 1:
addition of H+ electrophile to pi bond
- step 2:
addition of H2O nucleophile to cation
Hydration Mechanism
Halogenation of Alkenes
CH2=CH2 + Cl2 ---> Cl-CH2-CH2-Cl
- mechanism:
Cl2 is an electrophile (adds Cl+)
then Cl- is a nucleophile
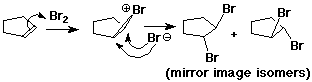
Anti Addition
- anti stereochemistry:
two new groups are added to opposite sides of the original pi bond
cyclopentene + Br2 ---> trans-1,2-dibromocyclopentane (no cis)
- anti - describes the process
- trans - describes the product
Bromonium Ion
- carbocations can be stabilized by bonding to a neighboring Br
(also works with Cl, but less favorable)
Reduction of Alkenes
- reduction - addition of H2
(or removal of O)
CH2=CH2 + H2 ---> CH3-CH3
R-O-H + H2 ---> R-H + H2O
Catalytic Hydrogenation
CH2=CH2 + H2 ---> CH3-CH3
- requires an active catalyst, typically Pt, Pd, Ni, PtO2
- reaction occurs on the surface
- both Hs are delivered to the same side of the pi bond
Syn Addition
- syn stereochemistry: two new groups are added to the same side of the
original pi bond
1,2-dimethylcyclohexene + H2 --(cat)-->cis-1,2-dimethylcyclohexane(no
trans)
- syn - describes the process
- cis - describes the product
Oxidation of Alkenes
- oxidation - addition of O
(or removal of H2)
RCH2OH ---> RCH=O ---> RCOOH
- there are a wide variety of oxidizing agents:
O2, O3, KMnO4, CrO3, Na2Cr2O7
metals in high positive oxidation states
Hydroxylation
- alkene + KMnO4 --(base)--> 1,2-diol
- addition of two OH groups is syn
- cyclopentene --> cis-1,2-cyclopentanediol
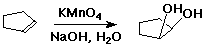
Oxidative Cleavage
- C=C --> C=O + O=C
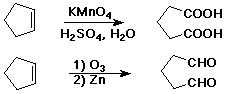
- acidic KMnO4 causes cleavage
- ozone (O3) causes cleavage
- sometimes useful degradation method to identify unknown compounds
Balancing Redox Reactions
- identify the two half-reactions
- what gets oxidized, what gets reduced?
- balance all elements except O and H
- balance O
use H2O (in acid) or OH- (in base)
- balance H
use H+ (in acid) or H2O (in base)
- balance charge with electrons (e-)
Polymers
- long chains of repeating units (monomers)
n CH2=CH2 --(init)--> (init)-(CH2-CH2)n-
n=100-10,000 polyethylene
- has properties like a very long alkane
- many polyalkenes are commercially important materials and plastics
e.g., PVC, Teflon, Orlon
Chain Reactions
- polymerization occurs by a free radical chain mechanism
- initiation - generation of the first free radical from an initiator
R-O-O-R --(heat)--> 2 R-O·
(initiators have one weak bond)
Chain Reactions
- propagation - radical adds to a p bond
RO· + CH2=CH2 ---> RO-CH2-CH2·
- note that the product is also a radical
RO-CH2-CH2· + CH2=CH2 ---> RO-CH2-CH2-CH2-CH2· --->
etc.
- typically this occurs hundreds or thousands of times
(until radicals recombine - termination)
Substituted Monomers
- radical additions follow the Markovnikov Rule:
add radicals to form the more stable radical intermediate
- radical stability is like cation stability: 3° > 2° >
1°
this leads to polymers with alternating substituents
Vinyl Polymers
- polyvinyl chloride
- polypropylene
- polystyrene
Alkyne Additions
- similar to alkenes but more reactive
- Markovnikov Rule is followed
- excess reagent gives double addition
- single addition is usually possible
- single addition gives alkene product, which may be cis (syn addition)
or trans (anti addition) or nonspecific
Reduction of Alkynes
- excess H2 + catalyst gives alkanes
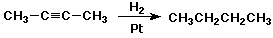
![]()
![]()
![]()


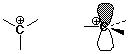



![]()