
Portland State University - - Professor Carl C. Wamser

Chapter 2 Notes - Alkanes & Cycloalkanes

Functional Groups
- a specific arrangement of atoms
- define chemical families
- determine chemical properties
- basis of nomenclature
- organization of textbooks
Hydrocarbons
- contain only C and H
- functional groups:
- only C-H and C-C single bonds (alkanes)
- C=C double bond (alkenes)
- C=C triple bonds (alkynes)
- rings (cycloalkanes)
- aromatic rings (arenes)
- form basic carbon skeleton
Heteroatoms
- Halogens ( X = F, Cl, Br, I )
- Oxygen ( C-O , C=O )
- Nitrogen ( C-N , C=N , C=N )
- Sulfur ( C-S , C=S )
- lots of other possibilities
- combinations of bonds
- other elements
Groups with Single Bonds
- R = general carbon group
- halides ( R-X )
- alcohols ( R-OH )
- ethers ( R-O-R )
- amines ( R-N )
- thiols ( R-SH )
- sulfides ( R-S-R )
Groups with Multiple Bonds
- carbonyl families ( C=O )
- imines ( C=N )
- nitriles ( C=N )
Carbonyl Families
- carbonyls:
aldehydes ( R-CO-H )
ketones ( R-CO-R )
- carboxyls:
carboxylic acids ( R-CO-OH )
esters ( R-CO-OR )
amides ( R-CO-NHR )
Alkane Family
- methane CH4
- ethane CH3CH3
- propane CH3CH2CH3
- butane CH3CH2CH2CH3
- alkane CH3(CH2)nCH3
- CnH2n+2 (homologous series)
Higher Alkanes
- pentane C5H12
- hexane C6H14
- heptane C7H16
- octane C8H18
- nonane C9H20
- decane C10H22
- note the Greek prefixes
Alkane Isomers
- carbon skeletons
- straight-chain
- branched-chain
- cyclic chain
- constitutional isomers
- atoms connected in a different order
- butane and isobutane
- 3 pentane isomers
Butane isomers
n-butane 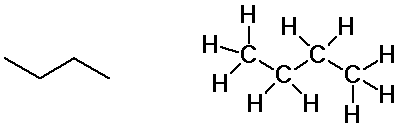
isobutane 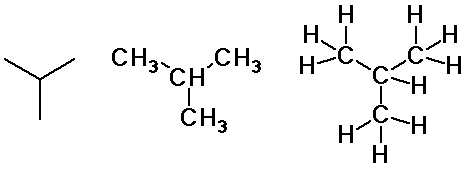
Pentane Isomers
- n-pentane
- isopentane
- neopentane
Alkyl Groups
n-propyl alcohol 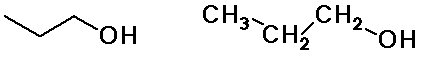
isopropyl alcohol 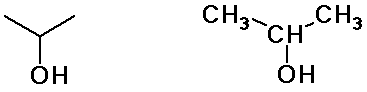
(constitutional isomers)
Butyl Groups
- n-butyl
- sec-butyl
- isobutyl
- tert-butyl
Classification of C Atoms
- 1° - primary - bonded to one other C
- 2° - secondary - bonded to 2 other C's
- 3° - tertiary - bonded to 3 other C's
- 4° - quaternary - bonded to 4 other C's
- class also applies to H atoms or functional groups attached to that
C
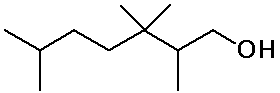
Identifying Carbon Classes
- identify the classes of each C, H and functional group in the molecule
below
IUPAC Nomenclature
(prefixes)-(parent alkane)-(suffixes)
(substituents)-(longest chain)-(family)
Cl-CH2CH2-OH
2-chloro ethan ol
IUPAC Rules
- parent = longest continuous carbon chain
- number chain from end closest to a substituent (1st difference)
- assign numbers to each subst.
- in naming, arrange substituents alphabetically
- for multiple substituents, use prefixes di-, tri-, tetra-, etc.
- but they don't count when alphabetizing
- separate names from numbers with hyphens, numbers from numbers with
commas
- otherwise all written as one word
IUPAC Examples
- name the three isomers of pentane
pentane
2-methylbutane
2,2-dimethylpropane
IUPAC Examples
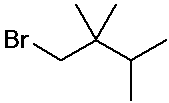
1-bromo-2,2,3-trimethylbutane
IUPAC Examples
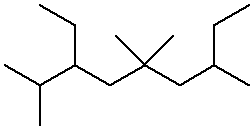
3-ethyl-2,5,5,7-tetramethylnonane
Conformations
- different 3D structures of the same molecule, differing only by rotations
about single bonds
Ethane Conformations
C-H bonds on the two carbons may or may not align
eclipsed: C-H bonds are aligned 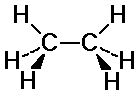
staggered: C-H bonds fit in between 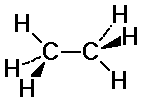
Newman Projections
- view down a C-C bond to visualize orientations
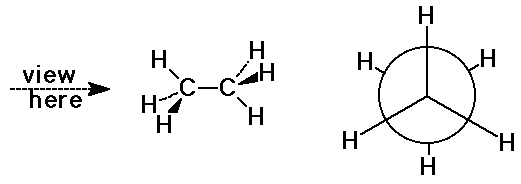
- staggered conformation (more stable)
Potential Energy Diagrams
- view energy changes as the C-C bond rotates
Writing Skeletal Structures
- omit C-H bonds
- assume C makes 4 bonds
- omit C atoms
- assume C at end of every bond
- especially useful for cyclic structures
- be able to put back all the details
Practice with Line Structures
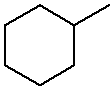 methylcyclohexane
methylcyclohexane
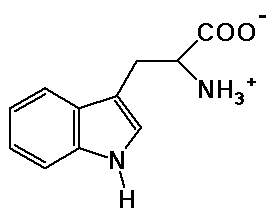 tryptophane
tryptophane
molecular formula is: C11H12N2O2
Cycloalkanes
- cyclo-prefix
- parent alkane is the ring size
- number so as to get lowest sum
- 1-methyl-3-propylcyclopentane
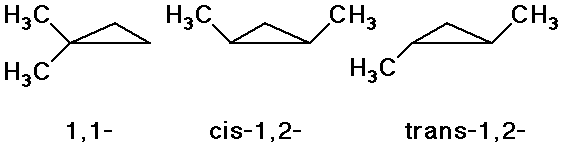
Cis-Trans Isomers
- stereoisomers: differ in 3D orientation, even though all atoms have
the same connections
- isomers of dimethylcyclopropane:
Small Rings
- typically unstable due to:
- angle strain
bent bonds
- eclipsing strain
- cyclopropane - planar
bond angles 60°
- cyclobutane - puckered
Common Rings
- cyclobutane - puckered
- cyclopentane - envelope
- cyclohexane - chair
Cyclohexane

- all bonds staggered
- all bond angles 109°
- practice drawing a good chair
opposite C-C bonds parallel
C-H bonds are straight up or down or parallel to a C-C bond
Substituted Cyclohexanes
- axial and equatorial positions
- the chair can flex
axial equilibrates with equatorial
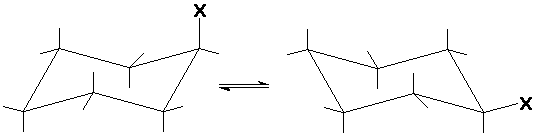
- equatorial is the preferred position
1,3-diaxial interferences (steric)
![]()
![]()
![]()

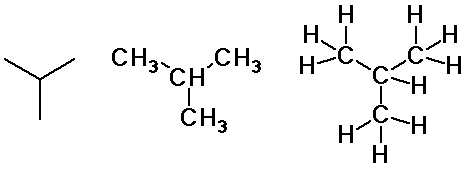
![]()
![]()
![]()
![]()

![]()


![]() methylcyclohexane
methylcyclohexane tryptophane
tryptophane

