![]()
![]()
![]()
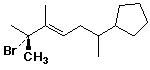
1. (2 points) Write a complete IUPAC name for the following compound, including a designation of stereochemistry:
2a. (2 points) Recopy the reaction below to clearly show the bond(s) that are broken and bond(s) that are made.
![]()
2b. (1 point) Identify each bond breaking as homolytic or heterolytic and each bond making as homogenic or heterogenic.
2c. (1 point) Identify nucleophiles and electrophiles.
2d. (1 point) Draw what could be a logical structure for the transition state for this reaction.
2e. (3 points) If the above reaction has an Ea of 15 kcal/mole and H = - 15 kcal/mole, draw what would be an appropriate reaction energy diagram. Label the axes, the reactants, the products, Ea, H, and the transition state. Is this reaction exothermic or endothermic?