

Professor Carl C. Wamser

Chapter 8 - Alcohols, Ethers, and Phenols
Alcohols, Ethers, Phenols
- functional groups:
- alcohol: C-O-H
- ether: C-O-C
- phenol: Ar-O-H
(Ar = aryl group)
Alcohol Classification
- based on the carbon the OH group is attached to:
1° , 2° , 3°
- methyl alcohol
CH3OH
- ethyl alcohol
CH3CH2OH (1°)
- isopropyl alcohol
(CH3)2CHOH (2°)
- t-butyl alcohol
(CH3)3COH (3°)
Alcohol Nomenclature
- OH group takes priority (even over -ene or -yne)
- it must be in the parent chain
- the direction of numbering gives it the lowest possible number
- -ol suffix with number designation
- name other substituents and multiple bonds as usual
Alcohol Examples
- common names for alcohols:
alkyl alcohol
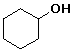
cyclohexyl alcohol or cyclohexanol

trans-4-methylcyclohexanol
Alcohol Example

(R)-3-methyl-5-hexen-3-ol
Phenol Nomenclature
- OH group assumed number 1
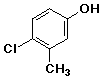
- 4-chloro-3-methylphenol
Ether Nomenclature
- alkyl alkyl ether (common)
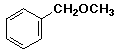
benzyl methyl ether
- alkoxy substituent (IUPAC)
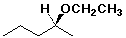
(S)-2-ethoxypentane
Hydrogen Bonding
- sp3 O with two covalent bonds and two lone pairs
- O lone pairs attract polar H bonds
- covalent O-H bond strength ~ 100 kcal/mole
- O...H (H-bond) strength ~ 5 kcal/mole
Effects of H-Bonding
- alcohols have higher boiling points than alkanes (nonpolar)
or alkyl halides (polar, but no H-bonds)
- ethers are polar but have no H-bonds
(pentane and diethyl ether both boil at about 35°)
- H-bonds hold together the strands of DNA ("velcro" effect)
Acid-Base Reactions
- remember analogy with water
- reactions as bases:
H2O + H+ <==> H3O+
ROH + H+ <==> ROH2+ (an oxonium ion)
- reactions as acids:
H2O + B- <==> B-H + OH-
ROH + B- <==> B-H + RO- (an alkoxide ion)
Acidity of Alcohols
- alcohols about as acidic as water
MeOH more acidic, EtOH less acidic
3° alcohols much weaker acids
- pKa values: 3° > 2° > 1° > MeOH
18 , 17, 16, 15.5 (compare H2O: pKa = 15.7)
- tBuOH + NaOH ---> unfavorable
Alkoxide Anions
- deprotonation of alcohols gives alkoxide anions
CH3OH + NaNH2 ---> NH3 + CH3O- Na+ (sodium methoxide)
- most commonly made by direct reaction with active metals
CH3OH + Na ---> 1/2 H2 + CH3O- Na+
(CH3)3COH + K ---> 1/2 H2 + (CH3)3CO-K+
Acidity of Phenols
- phenoxide anions are stabilized by resonance with the aromatic ring
- pKa of phenol is 10
(deprotonated by NaOH at pH 10)
- electron withdrawing groups further stabilize the phenoxide anion
(especially ortho or para)
- pKa of p-nitrophenol is 7
Oxygen Functional Groups
- alcohols are just the first of the many possible oxygen functional
groups
- oxidation leads to increasing number of bonds to oxygen
alkane --> alcohol --> carbonyl --> carboxyl --> CO2
- reduction leads to decreasing number of bonds to oxygen
CO2 --> carboxyl --> carbonyl --> alcohol --> alkane
Synthesis of Alcohols
- hydration of alkenes
follows Markovnikov's Rule
1-hexene --(H+, H2O)--> 2-hexanol
- reduction of carbonyl and carboxyl compounds
reducing agents:
sodium borohydride (NaBH4)
lithium aluminum hydride (LiAlH4)
Alcohol Redox Reactions
- reductions to prepare alcohols:
- aldehydes or ketones plus NaBH4
- carboxylic acids or esters plus LiAlH4
- oxidations of alcohols:
- 1° alcohol to aldehyde with PCC
- 2° alcohol to ketone with CrO3
- 1° alcohol to carboxylic acid with CrO3
Alcohol Redox Examples

Synthesis of Ethers
- Williamson ether synthesis
an SN2 reaction with an alkoxide as nucleophile
the alkyl halide should be methyl or 1°
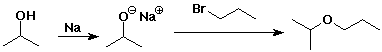
Reactions of Alcohols
- acid/base reactions
- oxidation reactions
- elimination (dehydration)
- substitution (C-O bond cleavage)
Substitution Reactions
- substitution (and elimination)
OH is a poor leaving group
but initial protonation creates a good leaving group (H2O)
- dehydration (E1 mechanism)
- halide substitution (SN1)
tBuOH + HBr --> tBuOH2+ --> tBu+ --> tBuBr
Ether Reactions
- ethers are generally unreactive
(make good solvents)
- react with strong acid
(protonated form can undergo SN1 or SN2 substitution)
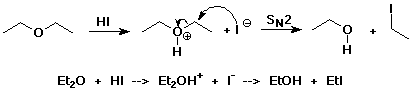
Phenol Reactions
- the phenol C-O bond is rarely cleaved
(SN1, SN2, E1, and E2 don't work for aryl groups)
- phenoxide anions are good nucleophiles
- electrophiles add readily to the benzene ring
OH is activating and an o,p-director
Quinones
- phenols are readily oxidized to quinones
- reduction of quinones gives a hydroquinone
a common reversible redox pair
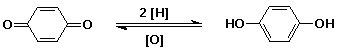
Epoxides
- cyclic 3-membered ring ethers
- named as 1,2-epoxyalkane
- prepared from alkenes
- unlike other ethers, these react easily to undergo ring opening
Epoxide Reactions
- acid-catalyzed hydration
- base-catalyzed hydration
- product is trans diol
(backside attack as in the reaction of bromonium ions)

Sulfur Functional Groups
- thiols: C-S-H group
(analogous to alcohols)
- sulfides: C-S-C group
(analogous to ethers)
- disulfides: C-S-S-C group
(analogous to peroxides)
- similar to oxygen analogs except:
better nucleophiles
easier to oxidize
![]()
![]()
![]()




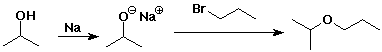
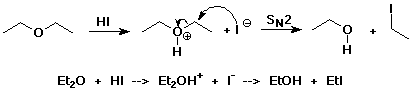
![]()
