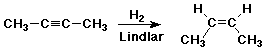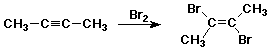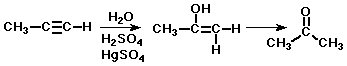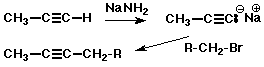Professor Carl C. Wamser

Chapter 4 - Alkenes and Alkynes
Alkene Addition Reactions
- pi bonds undergo addition reactions
- CH2=CH2 + HCl --> CH3CH2Cl
- in general,
- C=C + HX --> H-C-C-X
- alkenes react with hydrogen halides to form alkyl halides
Addition of HX to Alkenes
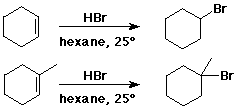
- cyclohexene + HBr --> bromocyclohexane
- 1-methylcyclohexene + HBr --> 1-bromo-1-methylcyclohexane (not 1-bromo-2-methylcyclohexane)
Reaction Notation
- reactants -------> products
- focus on the organic reactants and products
- show reagents over the arrow
- show solvent and conditions under the arrow
- (or show full balanced reaction)
Orientation of Addition
- regiochemistry:
- specific orientation of addition
- (which C gets H, which gets X?)
- alkene additions are regioselective:
- one direction of addition is usually preferred
Markovnikov's Rule
- the original:
- add H to the C with more H's
- (or to the C with fewer alkyl groups)
- the reason:
- add H+ to form the more stable cation
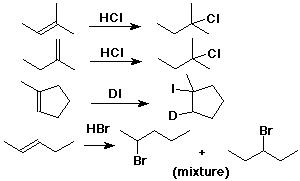
- CH3CH=CH2 + HCl --->
CH3CH+CH3 (not CH3CH2CH2+)
---> CH3CHClCH3 (not CH3CH2CH2Cl)
Tues, Feb. 13
Carbocations
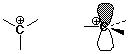
- structure: trigonal (sp2)
- stability: 3° > 2° > 1°
- more alkyl groups stabilize a cation by electron donation to the electron-deficient
(6-electron) carbocation
Markovnikov Addition
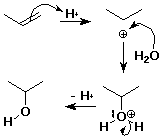
Hydration of Alkenes
- alkene + water --> alcohol
- CH2=CH2 + H2O --(H+)--> CH3CH2OH
- mechanism:
- step 1:
addition of H+ electrophile to pi bond
- step 2:
addition of H2O nucleophile to cation
Hydration Mechanism
Halogenation of Alkenes
- CH2=CH2 + Cl2 ---> Cl-CH2-CH2-Cl
- mechanism:
- Cl2 is an electrophile (adds Cl+)
- then Cl- is a nucleophile
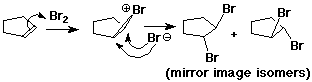
Anti Addition
- anti stereochemistry: two new groups are added to opposite sides of
the original pi bond
- cyclopentene + Br2 ---> trans-1,2-dibromocyclopentane (no cis)
- anti - describes the process
- trans - describes the product
Bromonium Ion
- carbocations can be stabilized by bonding to a neighboring Br
- (also works with Cl, but less favorable)
Reduction of Alkenes
- reduction - addition of H2
- (or removal of O)
- CH2=CH2 + H2 ---> CH3-CH3
- R-O-H + H2 ---> R-H + H2O
Catalytic Hydrogenation
- CH2=CH2 + H2 ---> CH3-CH3
- requires an active catalyst, typically Pt, Pd, Ni, PtO2
- reaction occurs on the surface
- both Hs are delivered to the same side of the pi bond
Syn Addition
- syn stereochemistry: two new groups are added to the same side of the
original pi bond
- 1,2-dimethylcyclohexene + H2 --(cat)-->cis-1,2-dimethylcyclohexane(no
trans)
- syn - describes the process
- cis - describes the product
Oxidation of Alkenes
- oxidation - addition of O
- (or removal of H2)
- RCH2OH ---> RCH=O ---> RCOOH
- there are a wide variety of oxidizing agents:
- O2, O3, KMnO4, CrO3, Na2Cr2O7
- metals in high positive oxidation states
Hydroxylation
- alkene + KMnO4 --(base)--> 1,2-diol
- addition of two OH groups is syn
- cyclopentene --> cis-1,2-cyclopentanediol
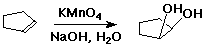
Oxidative Cleavage
- C=C --> C=O + O=C
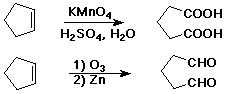
- acidic KMnO4 causes cleavage
- ozone (O3) causes cleavage
- sometimes useful degradation method to identify unknown compounds
Polymers
- long chains of repeating units (monomers)
- n CH2=CH2 --(init)--> (init)-(CH2-CH2)n-
- n=100-10,000 polyethylene
- has properties like a very long alkane
- many polyalkenes are commercially important materials and plastics
- e.g., PVC, Teflon, Orlon
Chain Reactions
- polymerization occurs by a free radical chain mechanism
- initiation - generation of the first free radical from an initiator
- R-O-O-R --(heat)--> 2 R-O·
- (initiators have one weak bond)
Chain Reactions
- propagation - radical adds to a p bond
- RO· + CH2=CH2 ---> RO-CH2-CH2·
- note that the product is also a radical
- RO-CH2-CH2· + CH2=CH2 ---> RO-CH2-CH2-CH2-CH2· --->
etc.
- typically this occurs hundreds or thousands of times
- (until radicals recombine - termination)
Substituted Monomers
- radical additions follow the Markovnikov Rule:
- add radicals to form the more stable radical intermediate
- radical stability is like cation stability: 3° > 2° >
1°
- this leads to polymers with alternating substituents
Vinyl Polymers
- polyvinyl chloride
- polypropylene
- polystyrene
Elimination Reactions
- alkenes are typically prepared by elimination reactions
- loss of HX from alkyl halides
- (promoted by strong base)
- loss of H2O from alcohols
- (promoted by strong acid)
- eliminations are the reverse of additions
Dehydrohalogenation
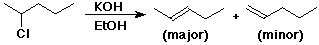
Dehydration

Zaitsev Rule
- predicts regiochemistry
- the major product in an elimination reaction is the more substituted
alkene
- (generally more stable)
Conjugated Dienes
- two double bonds separated by one single bond
- overlap their p orbitals into an extended (conjugated) molecular orbital
- more stable than separate pi bonds
- e.g., 1,3-butadiene

1,2- and 1,4-Additions
- electrophilic additions (HX, X2, etc.) often add at opposite ends (1,4)
of a conjugated diene
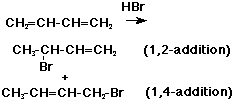
Allylic Carbocations
- initial electrophilic addition occurs at end (not middle) of a conjugated
diene
- the resultant cation retains three overlapping p orbitals (stabilized)
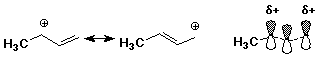
- allyl - position next to a C=C bond
- (vinyl - position on a C=C bond)
Allylic Resonance
- allylic cation has two Lewis structures (resonance forms)
- the actual structure is a hybrid
- the allyl cation is more stable than normal alkyl cations (due to resonance)
Resonance Forms
- each resonance form is a correct Lewis structure
- no atoms change (only electrons)
- equivalent resonance forms are most important for stabilization
- nonequivalent resonance forms may also contribute
- actual structure is a resonance hybrid
Alkynes - Structure
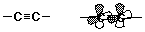
- carbon-carbon triple bond
- sp hybridization (linear)
- no cis-trans possibilities
- the two pi bonds are perpendicular
- high electron density
- (usually more reactive than alkenes)
Alkynes - Nomenclature
- -yne suffix (with number)
- rules similar to alkenes
- with both -enes and -ynes, suffix is -enyne and numbering is from the
end closer to a multiple bond
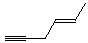
- (E)-4-hexen-1-yne
Alkyne Additions
- similar to alkenes but more reactive
- Markovnikov Rule is followed
- excess reagent gives double addition
- single addition is usually possible
- single addition gives alkene product, which may be cis (syn addition)
or trans (anti addition) or nonspecific
Reduction of Alkynes
- excess H2 + catalyst gives alkanes
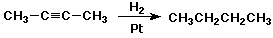
- Lindlar catalyst gives cis-alkenes
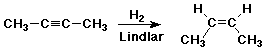
Halogenation of Alkynes
- first addition of X2 is anti
- product is trans-dibromoalkene
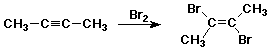
Hydration of Alkynes
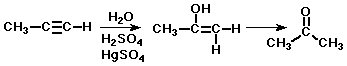
- initial product is an enol, which is typically unstable
- an enol isomerizes to a ketone
- (tautomers - a special kind of isomer, where the only difference is
the placement of one hydrogen)
Alkyne Acidity
- terminal alkynes have a C-H bond which is slightly acidic
- pKa ~ 25 (for CH2=CH2 , pKa ~44)
- NaNH2 is a strong enough base to deprotonate 1-alkynes
- anions are called acetylide ions, strong bases and good nucleophiles
Alkyne Syntheses
- acetylide anions are useful in preparing larger alkynes
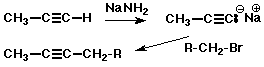
![]()
![]()

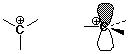



![]()
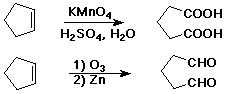
![]()
![]()
![]()
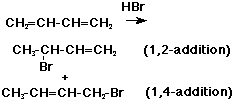
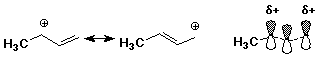
![]()