
 Professor Carl C. Wamser
Professor Carl C. Wamser

Chapter 3 - Alkenes. Organic Reactions
Alkenes
- C=C double bond
- CnH2n general formula
- unsaturated
not "saturated" with maximum hydrogens
unsaturated fats have some double bonds (easier to digest)
- cycloalkanes also are CnH2n
Alkene Nomenclature
- parent alkene is the longest continuous carbon chain that includes
the double bond
- number from the end that gives the double bond the lower number
- use -ene suffix and only the first number of the double bond
- name substituents as usual
Cycloalkene Nomenclature
- double bond assumed at C1-C2
- number in the direction that gives substituents lower numbers
 3-methylcyclohexene
3-methylcyclohexene
 5,5-dimethyl-1,3-cyclopentadiene
5,5-dimethyl-1,3-cyclopentadiene
Alkene Structure
- C=C double bond is one sigma bond and one pi bond
- sp2 hybridization (trigonal planar)
- pi bond doesn't rotate
- unlike ethane, ethene has no other conformers
Cis-Trans Isomers
- another example of stereoisomers
- 2-butene has two isomers:
 trans-2-butene
trans-2-butene
 cis-2-butene
cis-2-butene
- usually trans is more stable than cis
Cis-Trans Isomers
- an alkene can have cis-trans isomers only when each C in the double
bond is attached to 2 different groups
Stereochemistry Designation
 cis or trans ?
cis or trans ?
- note that cis-trans works only for disubstituted alkenes
- E,Z designation is more general
E,Z Designation
- assign priorities to the two groups on each C of the double bond (high,
low for each C)
- if the the two high priority groups are on the same side, it is Z
- if the the two high priority groups are on opposite sides, it is E
Sequence Rules
- assign priority based on the atoms directly attached to the carbons
of the double bond
- higher priority goes to:
- higher atomic number
- break ties by considering the next bonded atoms
Practice Nomenclature
 (E)-3,4-dimethyl-2-octene
(E)-3,4-dimethyl-2-octene
 (Z)
(Z)
Reaction Types
- additions
A + B --> C
H-Cl + CH2=CH2 --> CH3-CH2-Cl
- eliminations
X --> Y + Z
CH3-CH2-Cl --> CH2=CH2 + H-Cl
- substitutions
A-B + C-D --> A-C + B-D
CH4 + Cl2 --> CH3-Cl + H-Cl
- rearrangements
X --> Y
cyclopropane --> propene
Reaction Mechanisms
- a step-by-step account of how a reaction occurs (and why)
- bond-breaking steps:
homolytic: one electron to each fragment
(generates radicals)
heterolytic: both electrons to one fragment
(generates ions)
- bond-making steps:
homogenic or heterogenic
Electron Movement
- electron-pushing arrows indicate the flow of electrons in a mechanism
- electrophile:
electron-deficient species seeking a pair of electrons (a Lewis acid )
- nucleophile:
electron-rich species that can provide a pair of electrons (a Lewis base)
HCl plus Ethene
- CH2=CH2 + H-Cl --> CH3-CH2-Cl
- an electrophilic addition
- reaction type: addition
- reagent type: an electrophile
HCl, actually H+, a strong Lewis acid
Addition Mechanism
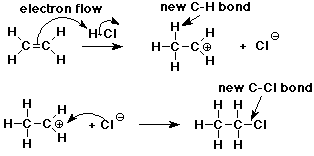
- pi bond is relatively reactive, especially towards electrophiles
- it provides a good source of electrons
- addition of H+ to CH2=CH2 forms a new C-H sigma bond
- the electrons for the new bond came from the pi bond
- the other C is left with only 6 e-
Carbocation Intermediate
- an intermediate is formed in the reaction mechanism
- CH2=CH2 + H+ --> CH3-CH2+
- carbocation: a carbon atom with only 3 bonds (6 e-) and a positive
charge
- structure: sp2 hybridized (trigonal)
Formation of Chloroethane
- the reaction is completed as chloride anion (a nucleophile) adds to
the carbocation (an electrophile)
- CH3-CH2+ + Cl- ---> CH3-CH2-Cl
Equilibrium
- for a general reaction:
A + B <==> C + D
- equilibrium constant, K
K = [C] [D] / [A] [B]
- favorable reactions have large K
- unfavorable reactions have small K
Kinetics
- rates of reaction
- may or may not correlate with the favorability of the equilibrium
- rate depends on the mechanism
- is there a good way to get from reactants to products?
Heats of Reaction
- delta H (enthalpy change)
delta H = H(products) - H(reactants)
- exothermic means delta H < 0 (negative)
reaction gives off heat
- endothermic means delta H > 0 (positive)
reaction absorbs heat
Potential Energy Diagrams
- draw exothermic reactions downhill
- draw endothermic reactions uphill
Activation Energy, Ea
- there is usually an energy barrier between reactants and products
- activation energy represents the highest amount of energy necessary
while travelling along the minimum-energy (easiest) pathway from reactants
to products
The Transition State
- structure of the molecule(s) at the highest point along the reaction
pathway
- the stability of the transition state (relative to reactants) determines
Ea (rate of reaction)
![]()
![]()
![]() 3-methylcyclohexene
3-methylcyclohexene ![]() 5,5-dimethyl-1,3-cyclopentadiene
5,5-dimethyl-1,3-cyclopentadiene  trans-2-butene
trans-2-butene  cis-2-butene
cis-2-butene  cis or trans ?
cis or trans ? (E)-3,4-dimethyl-2-octene
(E)-3,4-dimethyl-2-octene  (Z)
(Z) 