![]()
![]()
Write out all the possible products that could be formed by monochlorination of methylcyclobutane.
Complete each of the following reactions:

Describe the ways you could experimentally distinguish whether a particular substitution reaction followed an SN1 or an SN2 pathway.
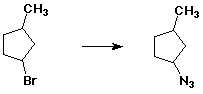
Describe the ways you could experimentally distinguish whether a particular elimination reaction followed an E1 or an E2 pathway.
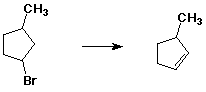
Write potential energy diagrams that distinguish the different pathways.
Write the transition states for the rate-determining step of each pathway.
Predict and/or control what a given compound is likely to do under
certain circumstances
SN1 |
SN2 |
E1 |
E2 | |
CH3X |
No |
good nucl. |
No |
No |
1° ( RCH2X ) |
No |
good nucl., |
No |
strong base, |
2° (R2CHX ) |
No |
good nucl., |
No |
strong base |
3° ( R3CX ) |
good nucl., |
No |
polar solvent, |
strong base |
Good nucl., |
Good nucl., |
Poor nucl., |
Poor nucl., | |
CH3X |
SN2 |
SN2 |
SN2 |
No reaction |
1° ( RCH2X ) |
SN2 |
SN2 |
E2 |
No reaction |
2° (R2CHX ) |
E2 |
SN2 |
E2 |
No reaction |
3° ( R3CX ) |
E2 |
SN1 |
E2 |
SN1 |