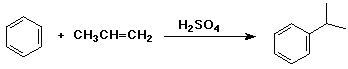Chapter 5 - Aromatic Compounds
Practice Exercises

Resonance Stabilization and Aromaticity
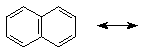
one resonance form of naphthalene is shown below - write all the others
that involve simply shifting double and single bonds (i.e., no charges,
all carbons with four bonds)
electrophilic aromatic substitution on naphthalene gives the carbocation
intermediate shown below - write all other resonance forms
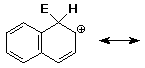
write a structure for the resonance hybrid that shows the positions where
the positive charge is expected
Electrophilic Aromatic Substitution - Mechanism
any reaction that could generate a carbocation could initiate a Friedel-Crafts
substitution reaction
shown below is an industrial synthesis that makes cumene from benzene and
propene
note that there are no side products and H2SO4 is a catalyst (recycles)
- these are important considerations in an economic industrial process
write a complete mechanism, including all resonance forms for the intermediate
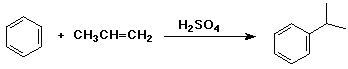
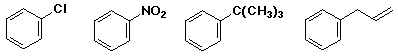
Reactions of Aromatic Compounds
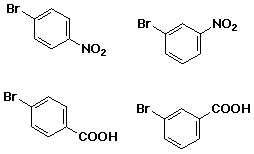
write the reactions you would use to make each of the following from
benzene:
Substituent Effects
both substituents shown below are ester functional groups, yet one acts
as a meta-director and one as an ortho, para-director - explain
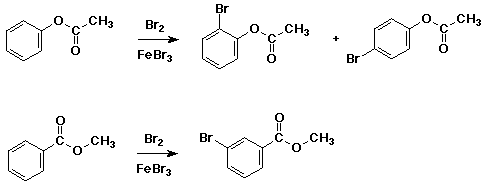
which of the reactions above would you predict to be faster?
Synthesis
starting from benzene, develop synthetic sequences for each of the following: