![]()
McMurry, pp 167 - 170:
Problems 5.22-24, 26-29, 35-40, 43, 44, 49, 50
1. Write complete names for each of the following compounds:
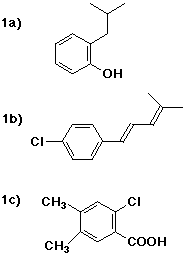
1a) o-isobutylphenol
1b) (1E)-1-(p-chlorophenyl)-4-methyl-1,3-pentadiene
1c) 2-chloro-4,5-dimethylbenzoic acid
2. Apply the Markovnikov Rule to the addition of HBr to styrene (phenylethene).
For the predicted intermediate cation, write all the resonance forms.
The electrophile (H+) will add to the end of the pi bond and form
a benzylic cation that is stabilized by resonance in a manner similar to
an allylic cation.

3. Consider whether the nitroso group (-N=O) would be activating or deactivating,
and whether it would be ortho,para or meta directing. Justify your choices.
It is an activating o,p-director.
The lone pair on N can be donated to the intermediate cation to stabilize
the positive charge by resonance.
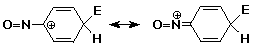
A good case could also be made for -N=O as electron-withdrawing since O
is more electronegative than N and the N=O bond is polarized with N partially
positive.