![]()
McMurry, pp 137 - 140:
Problems 4.25-31, 33, 34, 36, 39, 40, 41, 43, 44, 45, 46, 47, 53, 54, 55
1. Write IUPAC names, including designation of stereochemistry where needed,
for each of the following compounds:
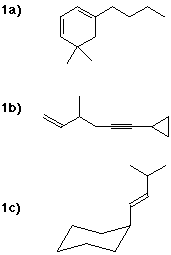
1a) 1-butyl-5,5-dimethyl-1,3-cyclohexadiene
1b) 6-cyclopropyl-3-methyl-1-hexen-5-yne
1c) (E)-1-cyclohexyl-3-methyl-1-butene
(in its axial conformation)
2. When chlorine is added to an alkene in aqueous (water) solution, another
major product besides the dichloro addition product is usually observed.
For example, propene reacts with aqueous chlorine to form 1-chloro-2-propanol
as the major product.
Write a mechanism that explains how this product forms.
Indicate how formation of this product relates to the Markovnikov rule.
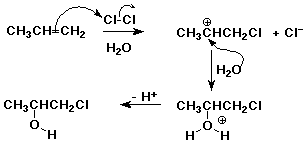
The reaction starts as usual, with the electrophile, Cl2, adding to the
double bond to form a cation intermediate. The nucleophile that adds to
the cation can be water, because of its abundance, as well as chloride (Cl-).
The usual Markovnikov Rule doesn't apply because we are not adding HX. However,
remembering the reasoning behind the Markovnikov Rule, the electrophile
should add so as to give the more stable cation. In this case that would
be 2° rather than 1°, and in fact the observation of 1-chloro-2-propanol
does indicate that the cation was at the 2-position, where water added.
3. Many alkenes undergo polymerization not only by free radical polymerization,
but also by cationic polymerization. During World War 2, as a substitute
for natural rubber, butyl rubber was made by cationic polymerization of
isobutylene (2-methylpropene).
Write a chain reaction mechanism for cationic polymerization of isobutylene,
using strong acid (H+) as initiator.
Explain why isobutylene would be particularly favorable for this type of
reaction.
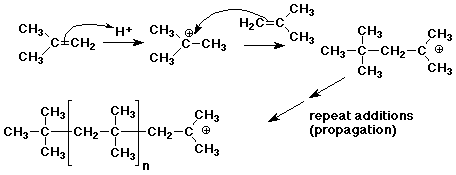
The initiator (H+) is a strong electrophile that adds to isobutylene to
form a relatively stable tert-butyl cation. This cation is also an electrophile
and can add to another isobutylene in a chain reaction. The propagation
steps are similar to those for free radical polymerization except that cations
rather than radicals are the intermediates in the growing chains. It works
well for isobutylene because the cations are tertiary and relatively stable.