![]()
McMurry, pp 137 - 140:
Problems 4.25-31, 33, 34, 36, 39, 40, 41, 43, 44, 45, 46, 47, 53, 54, 55
1. Write IUPAC names, including designation of stereochemistry where needed,
for each of the following compounds:
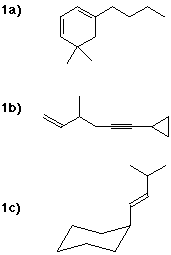
3. Many alkenes undergo polymerization not only by free radical polymerization,
but also by cationic polymerization. During World War 2, as a substitute
for natural rubber, butyl rubber was made by cationic polymerization of
isobutylene (2-methylpropene).
Write a chain reaction mechanism for cationic polymerization of isobutylene,
using strong acid (H+) as initiator.
Explain why isobutylene would be particularly favorable for this type of
reaction.