
Homework, Chapter 3 - Alkenes. Organic Reactions
McMurry, pp 97 - 102:
Problems 3.22, 23, 26, 27, 28, 29, 30, 32, 34, 35, 38, 39, 42, 48 - 55
1. Write IUPAC names, including designation of stereochemistry where needed,
for each of the following compounds:
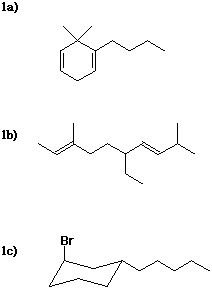
1a) 1-butyl-6,6-dimethyl-1,4-cyclohexadiene
1b) (2E,7E)-6-ethyl-3,9-dimethyl-2,7-decadiene
1c) trans-1-bromo-3-pentylcyclohexane
2. Imagine three different mechanisms for accomplishing the general substitution
reaction shown below. The mechanisms are described as:
R-X + Y:- ----> R-Y + X:-
a) all in one step
b) two steps: first loss of a leaving group, then attachment of a nucleophile
c) two steps: first bonding of the nucleophile, then loss of the leaving
group
Write stepwise mechanisms for each case and plausible potential energy diagrams.
*******
2a)
Y:- + R-X ---> [ Y....R....X ] ---> R-Y + X:-
potential energy diagram shows just one step
2b)
R-X ---> R+ + X:-
R+ + Y:- ---> R-Y
potential energy diagram shows two steps,
probably the first step endothermic and the second step exothermic
2c)
R-X + Y:- ---> Y....R....X
Y....R....X ---> R-Y + X:-
potential energy diagram shows two steps,
probably the first step endothermic and the second step exothermic
Note:
in 2a), the structure described as Y....R....X is a transition state
in 2b), the structure described as Y....R....X is an intermediate
![]()
