![]()
McMurry, pp 29 - 31:
Problems 1.27, 30, 31, 32, 38, 39, 41, 43, 45, 47, 49, 50, 55, 56
1. Write good Lewis structures for the compounds listed below. The structures
are written in the standard abbreviated forms, which give some indication
about the order in which atoms are bonded.
Indicate the hybridization of each atom and describe the expected geometry.
If there are additional reasonable resonance forms, show them and indicate
whether they contribute equally, or if not, which is the preferred resonance
form.
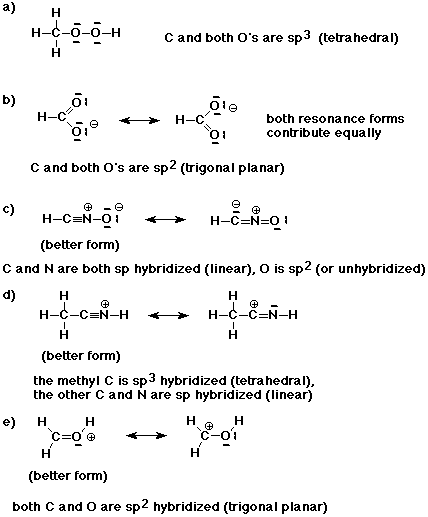
2. Given that Kw = [H+] [OH-] = 10E-14 , explain why the pKa of H2O is cited as 15.7 and the pKa of H3O+ as -1.7 .
In order to compare Ka in the same way as for other acids, the equilibrium constant should include concentration of water, [H2O]. So Ka = Kw / [H2O]. Since [H2O] in pure water is 55.5 M, Ka = 1.8 x 10E-16, or pKa = 15.7 .
The Ka for H3O+ would be defined as Ka = [H+] [H2O] / [H3O+] . But [H+] is the same as [H3O+] , so Ka = [H2O] = 55.5 , or pKa = -1.7 .
3. The pKa of CH3OH2+ (protonated methanol) is -2.2. Predict the preferred direction of an equilibrium acid-base reaction between H3O+ and CH3OH.
CH3OH + H3O+ <===> CH3OH2+ + H2O
The stronger acid is CH3OH2+ (more negative pKa), so the equilibrium will lie farther to the left.