![]()
![]()
![]()
1. (15 points) Write a complete IUPAC name for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) 
b) 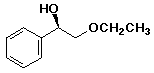
c) 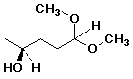
2. (15 points) Write accurate structures for the following:
a) benzyl isobutyl ether
b) sodium (S)-2-butoxide
c) an E2 transition state
d) a chiral epoxide
e) 3,4-dimethoxyphenol
3. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Indicate stereochemistry if it is specific.
a) ![]()
b) 
c)
d)
e)
4. (15 points) Arrange the following in order with respect to the property indicated. Write "MOST" under the most reactive or highest value and "LEAST" under the least reactive or lowest value.
a) reactivity in an SN2 mechanism
![]()
b) reactivity in an E1 dehydration
![]()
c) nucleophilicity
![]()
d) acidity
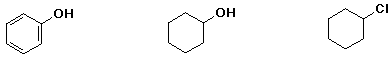
e) boiling point
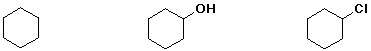
5. (15 points) Write a complete mechanism for the cleavage of dimethyl ether with excess HI to give methyl iodide plus water. Show all steps and use electron-pushing arrows to indicate bonds made and broken.
6. (10 points) Write a complete synthetic sequence to illustrate how you could prepare EITHER ONE of the compounds below. You may use as starting compounds any alcohol having four carbons or fewer.
Show each reaction step with the necessary reagents and conditions, but it is not necessary to show reaction mechanisms.
CHOOSE JUST ONE ![]()
7. (15 points) The 7D Mining Company needs some cyclohexanol, but they only have chlorocyclohexane (bottle A) and bromocyclohexane (bottle B) available.
Happy takes some A and adds water, but no reaction takes place.
Sleepy says that in an SN2 reaction Br- is a better leaving group than Cl-, so he takes some B and adds water, but again no reaction takes place.
Bashful says that water isn't a good enough nucleophile for an SN2 reaction, so he adds NaOH to Sleepy's reaction. A reaction does take place, and he isolates a new compound, but it isn't cyclohexanol.
Sneezy says that a Grignard reagent is even more reactive, so he adds magnesium to some B, observes a reaction, then adds water and some acid. He gets a reaction and also isolates a new compound, but it isn't the same compound that Bashful got, and it also isn't cyclohexanol.
Grumpy walks in and berates their chemistry skills. He grabs one of their compounds and runs a reaction that converts it into cyclohexanol.
a) Whose compound did he take and how did he turn it into cyclohexanol? Show the reactions.
b) Explain why none of the first four reactions offered a good chance to make cyclohexanol. What reactions did take place?