![]()
![]()
![]()
1. (15 points) Write a complete IUPAC name for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) 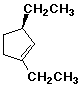
b) 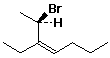
c) 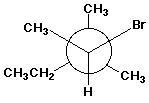
2. (10 points) Examine the pairs of compounds below and identify their relationship to one another. Use the letter codes below:
A - identical structures
B - constitutional isomers
C - conformational isomers
D - diastereomers
E - enantiomers
F - none of the above
a) 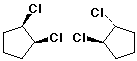
b) 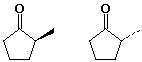
c) 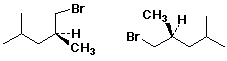
d) 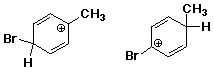
e) 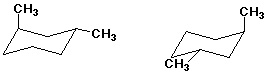
3. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Indicate stereochemistry if it is specific.
a) ![]()
b) ![]()
c) 
d) 
e) 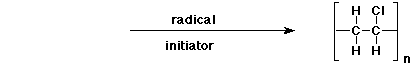
4. (15 points) Write all resonance forms for the cation intermediate that would be formed in the following steps.
a) 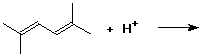
b) 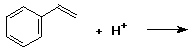
c) 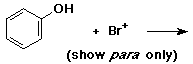
5. (15 points) Write a complete mechanism for the hydration of 1-methylcyclopentene
using aqueous sulfuric acid as catalyst.
Show all steps and use electron-pushing arrows to indicate bonds made and
broken.
6. (15 points) Write a complete mechanism for the Friedel-Crafts reaction
of chloroethane with benzene, using AlCl3 as catalyst.
Show each step and write all resonance forms for any intermediates involved.
7. (15 points) Write a complete synthetic sequence of reactions that
starts from benzene and creates m-bromobenzoic acid.
Show each reaction step with the necessary reagents and conditions, but
it is not necessary to show reaction mechanisms.