
Electrophilic Aromatic Substitution
- benzene can be made to react with very strong electrophiles
(E+)
- intermediate is a carbocation
(like addition to one of the pi bonds)
- nucleophiles don't add to the cation
(H+ leaves, regenerates benzene ring)
- reaction is substitution (E+ for H+)
Mechanism of Aromatic Substitution
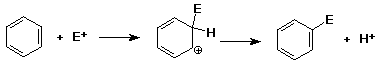
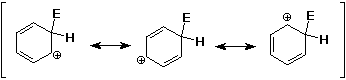
Mechanism - why slower than alkenes ?
- Ea for electrophilic attack on benzene is greater than Ea
for electrophilic attack on an alkene
- although the cation intermediate is delocalized and more
stable than an alkyl cation, benzene is much more stable than
an alkene
Mechanism - why substitution ?
- the substitution product regains the aromatic stability
- an addition product would be a conjugated diene, not as stable
Bromination of Benzene
- electrophile is Br+
- generated from Br2 + FeBr3
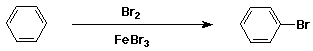
Chlorination of Benzene
- electrophile is Cl+
- generated from Cl2 + FeCl3
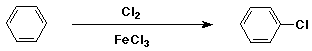
Nitration of Benzene
- electrophile is NO2+
- generated from H2SO4 + HNO3
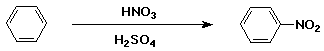
Sulfonation of Benzene
- electrophile is HSO3+
- generated from H2SO4 + SO3
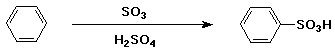
Friedel-Crafts Alkylation
- electrophile is an alkyl cation (R+)
- generated from RCl + AlCl3
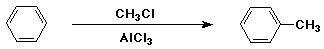
- limitations of the Friedel-Crafts reaction
- cation rearrangements may occur
- doesn't work with deactivated aromatic rings
- extensions of the Friedel-Crafts reaction
- other sources of cations, e.g., alkene + H+
Friedel-Crafts Acylation
- electrophile is an acyl cation (RCO+)
- generated from RCOCl + AlCl3
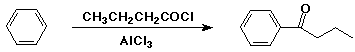
Substituent Effects
- substituents on the benzene ring can affect the reaction
in two ways:
reactivity - substituted benzene may react faster or slower
than benzene itself reacts
orientation - the new group may be oriented ortho, meta,
or para with respect to the original substituent
Reactivity Effects
- activating - reaction is faster
observed with electron-donating groups that make the ring more
electron-rich
- deactivating - reaction is slower
observed with electron-withdrawing groups that make the ring
less electron-rich
Orientation Effects
- substituent already present on the benzene ring determines
the location of the new group
- ortho,para-directors: electron-donating groups direct
the new group mainly to ortho & para
- meta-directors: electron-withdrawing groups direct
new group mainly meta
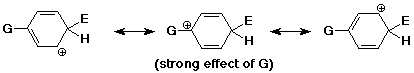
Ortho, Para Directors
- the best cation is formed when the electrophile adds either
ortho or para
(better than unsubstituted)
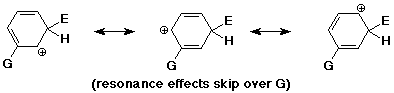
Meta Directors
- the best cation is formed when the electrophile adds meta
(but this is worse than unsubstituted)
Classifying Substituents
- activating and o,p-directing:
alkyl, aryl, O and N groups
- deactivating and m-directing:
N+ groups, polar multiple bonds
- deactivating but o,p-directing:
the halogens (F, Cl, Br, I)
(electron-withdrawing atoms, but lone pairs can stabilize the
cation when it is ortho or para)
Synthetic Strategy
- synthesis of complex compounds requires attention to the
order in which groups are attached
- retrosynthetic analysis - think backwards one step
at a time
(What reaction could have made this target compound?)
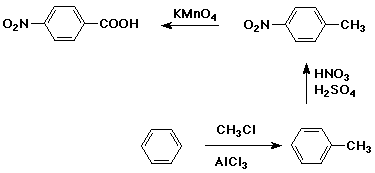
Synthesis Example
- target compound: p-nitrobenzoic acid
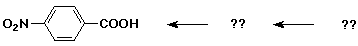
Synthesis Example
Nucleophilic Aromatic Substitution - Benzyne (Elimination/Addition)
- elimination from an aryl halide with a very strong base (usually
NaNH2 in NH3)
- intermediate is benzyne, highly reactive
- benzyne adds nucleophiles, e.g., NH3
- note different substitution patterns that can result

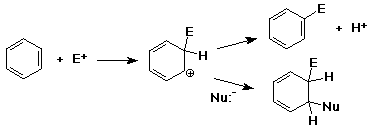
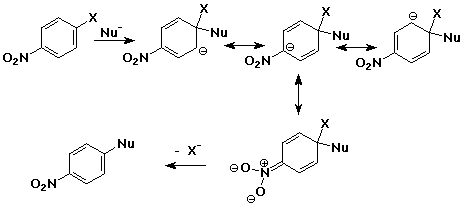
Nucleophilic Aromatic Substitution - Anion Intermediate
(Addition/Elimination)
- addition of nucleophile to an aryl halide ( at the ipso position
)
- intermediate is a delocalized anion, analogous to the cation
in electrophilc substitution
- usually workls only with strong electron-withdrawing groups
ortho & para (e.g., nitro)
- loss of leaving group returns the aromaticity



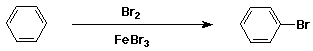
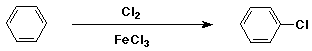
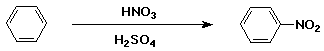
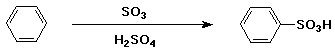
![]()
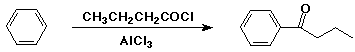


![]()


