Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring Term |
Quiz 27 |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring Term |
Quiz 27 |
![]()
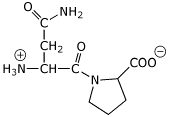
1. (2 points) Draw the structure of the dipeptide AsnPro as it would exist in solution at pH 7 (ignore stereochemistry).
2. (2 points) Draw the structures of the individual amino acids that would be formed after complete acid hydrolysis of GlnSer at pH 1 (including stereochemistry and appropriate ionization).
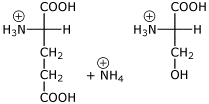
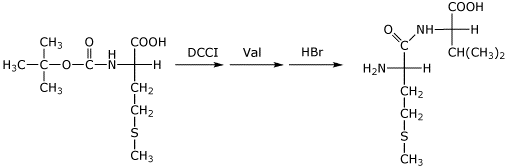
3. (3 points) Write a complete structure for BOC-protected methionine (with stereochemistry) and show the next steps that would be taken to prepare the dipeptide MetVal (write the structure of MetVal, stereochemistry not necessary).
4. (3 points) Identify the unknown hexapeptide based on the following data.
complete hydrolysis gives equal amounts of Arg, Asp, Gly, Ile, Lys, Tyr
trypsin hydrolysis generates three dipeptides
chymotrypsin hydrolysis releases Gly and a pentapeptide
Edman degradation releases Ile followed by ArgIle - Arg - Asp - Lys - Phe - Gly