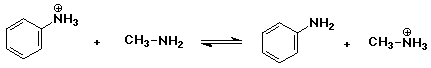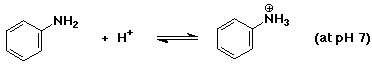|
|
Organic
Chemistry III
|
|
Professor
Carl C. Wamser
|
|
|
Chapter
22 Homework Answers
|

Carey, pages 991 - 1001 :
Problems 22.23 - 50
1. Write all the resonance forms for protonated imidazole. One is shown
below.
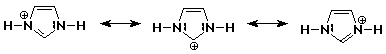
Note that the positive charge does not
show up on the other two carbon atoms
2. Predict whether the following acid-base equilibria would be more favorable
to the right or to the left. Use pKa data from your text.
a) 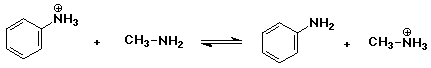
to the right (aliphatic amines are stronger bases than aromatic amines)
b) 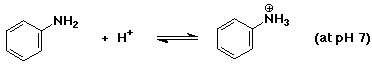
to the left (with a pKa about 4, it is neutral above pH 4)
c) 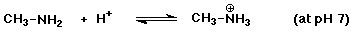
to the right (with a pKa about 10, it is protonated below pH 10)
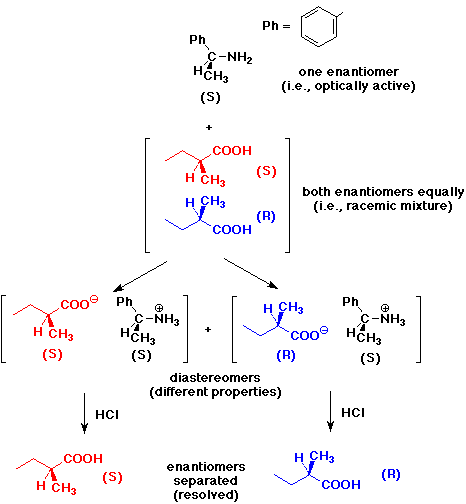
3. Given an optically active sample of 1-amino-1-phenylethane, write a
series of reactions that could be used to resolve a racemic mixture of 2-methylbutanoic
acid.


![]()