Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring Term |
Exam 2 |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring Term |
Exam 2 |
![]()
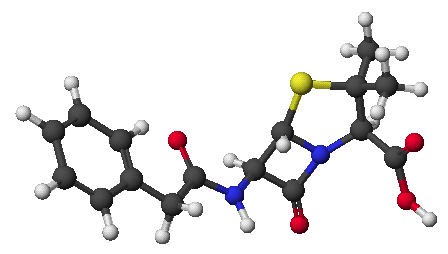
1. (15 points) Write complete names for each of the following:
a) 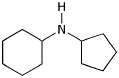
b) 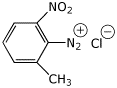
c) 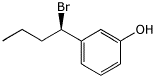
d) 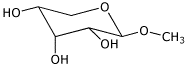
e) 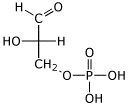
2. (15 points) Write complete structures for the following:
a) an L-aldotriose
b) 3-methylbenzyne
c) p-phenylbenzenediazonium tetrafluoroborate
d) alpha-D-fructofuranose
e) alpha-maltose (chair forms)
3. (15 pts) Arrange the following in order with respect to the property indicated. Write MOST and LEAST under the compounds with the highest and lowest values, respectively.
a) basicity
![]()
b) acidity
![]()
c) reactivity towards nucleophilic substitution
![]()
d) number of stereocenters
![]()
e) number of formic acid equivalents that would be released from complete HIO4 oxidation
![]()
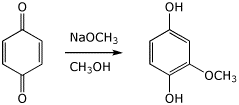
4. (15 points) Complete the following reactions by adding the missing part, either the necessary reagents and conditions or the final major product.
a) ![]()
b) 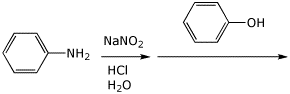
c) 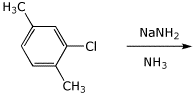
d) ![]()
e) ![]()
5. (10 points) Show how to synthesize the target compound below, starting from benzene.
Your pathway should include p-nitrophenol as an intermediate product.
![]()
6. (16 points) Write complete mechanisms for each of the following reactions, including resonance forms for intermediates. Electron-pushing arrows are optional.
a) ![]()
b) (Hint - think Michael addition and keto-enol tautomerism)
7. (14 points) D-Glucosamine has the same structure as D-glucose, except C-2 is a primary amine instead of an alcohol (with the same stereochemistry).
Write the structure of D-glucosamine
(3 points) in an open-chain form
(3 points) as a beta-pyranose using a Haworth form
If D-glucosamine is treated with sodium nitrite and acid, two epimeric aldohexoses are formed.
(4 points) Write these two aldohexoses in open-chain form.
The main component of chitin is a polymer analogous to cellulose (beta 1-4 linkages), but made from D-glucosamine in which the amine group is converted to an acetamide.
(4 points) Write the structure of this polymer (at least two units) using chair forms.