Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring Term |
Exam 1 Answer Key |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring Term |
Exam 1 Answer Key |
![]()
1. (15 points) Write complete names for each of the following:
a) 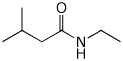
b) 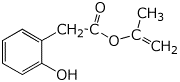
c) 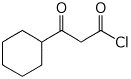
d) ![]()
e) 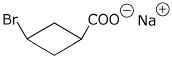
2. (15 points) Write complete structures for the following:
a) 2,4,5-trichlorobenzonitrile
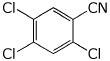
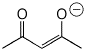
b) the enolate anion (best resonance form) of 2,4-pentanedione
c) the Claisen condensation product from ethyl propanoate
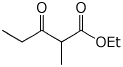
d) p-methylbenzyl p-methoxybenzoate
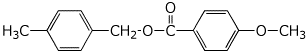
e) malonic anhydride
![]()
3. (15 points) Complete the following reactions by adding the missing part, either the necessary reagents and conditions or the final major product.
a) 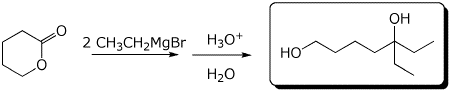
b) 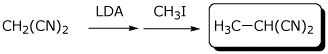
c) 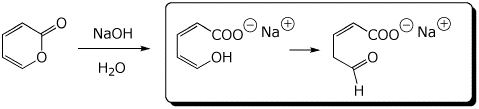
d) 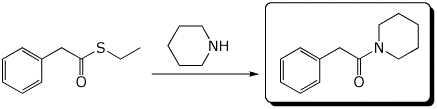
e) 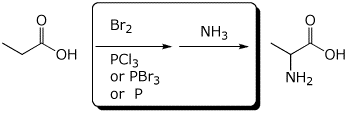
4. (15 points) Write a complete mechanism for the acid-catalyzed reaction below.
Show all steps and all resonance forms for any intermediates involved.
You may use H+ and -H+ to show proton transfer steps.
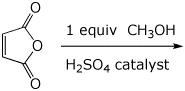
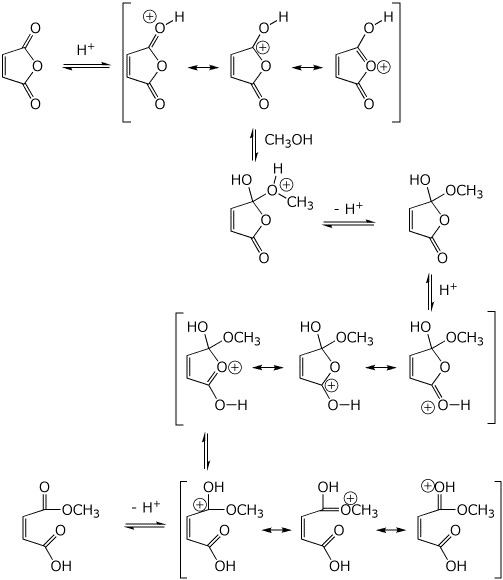
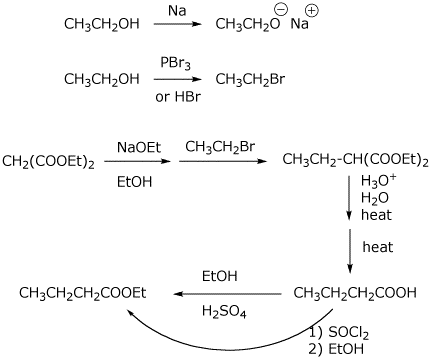
5. (20 points) Show how to synthesize the compound below. EVERY carbon-containing compound you use must come from either malonic ester or ethanol.
![]()
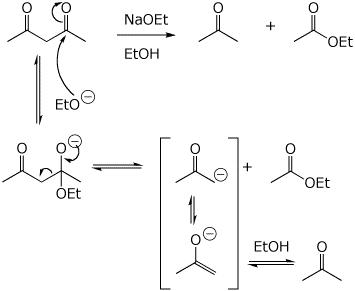
6. (20 points) The Claisen condensation is fully reversible. Illustrate the reversal of a Claisen condensation with the compound shown below. Write a complete mechanism, including electron-pushing arrows.
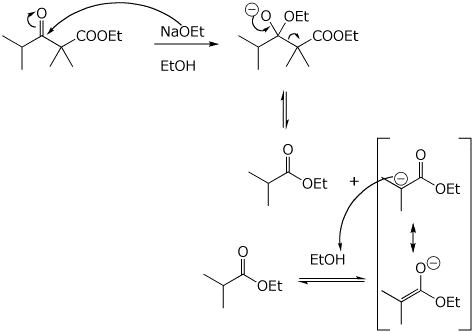
In a similar manner, beta-diketones can be cleaved at their alpha position, as shown below.
Write a complete mechanism, including electron-pushing arrows.