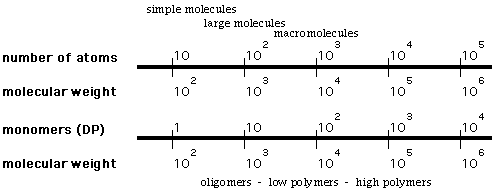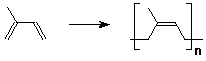|
|
Organic
Chemistry III
|
|
Professor
Carl C. Wamser
|
|
|
Chapter
29 Notes
|

Polymers

A. Polymers - macromolecules
- polymers are abundant and important, both naturally and synthetically
- estimate up to 50% of practicing chemists work on polymers
- polymer chemistry encompasses all other areas of chemistry:
organic, inorganic, physical, biological
Examples of typical polymers:
- inorganic: natural
sand
clays
- inorganic: synthetic
fiberglass
ceramics
- organic: natural (biopolymers)
polysaccharides (wood, starch)
polypeptides (proteins)
polyisoprenes (natural rubber)
nucleic acids (RNA, DNA)
- organic: synthetic
polyolefins
polyesters, polyamides
Special features of polymer chemistry:
1) SIZE
- molecular weights typically 10,000 - 1,000,000
- nonvolatile (tend to decompose at high temp.)
- chains become entangled easily (lots of conformations)
2) POLYDISPERSITY
- synthetic polymers always involve
a distribution of structures & molecular weights
- technically a mixture of molecules, although the properties
are virtually identical for all species
- must deal with average molecular weights
How big is a macromolecule?
- generally, a single molecule (held together by covalent bonds)
with at least several hundred atoms (molecular weight of several
thousand)
- special cases: aggregates of smaller molecules that behave
as a unit often have properties similar to macromolecules - e.g.
colloids, micelles, vesicles, liposomes
B. Terminology of Polymer Chemistry
polymer - a large molecule consisting of a number of
repeating units, with molecular weight typically several thousand
or higher
repeat unit - the fundamental recurring unit of a polymer
monomer - the smaller molecule(s) that are used to prepare
a polymer (may or may not be equivalent to the repeat unit)
oligomer - a molecule consisting of several repeat units
of a monomer, but not large enough to be considered a polymer
Approximate ranges for macromolecules, low polymers, high
polymers:
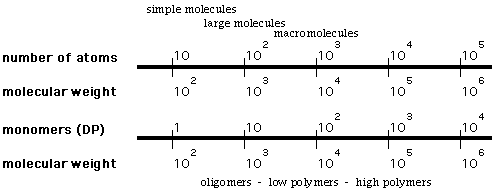
1. Classification of polymers:
copolymer - a polymer prepared from more than one monomer
addition polymer - a polymer that consists of a repeat
unit equivalent to its monomer - generally vinyl polymers, e.g.,
polyethylene, or ring-opening polymers, e.g., poly(ethylene oxide)
condensation polymer - a polymer that differs from its
monomer(s) by the elimination of a small molecule during polymerization
(Carothers' original definition) e.g., polyamides and polyesters
the definition has been expanded to include any polymer that
incorporates new functional group(s) in the chain that were not
present in the monomers
(to allow inclusion of polyurethanes)
2. Descriptions of polymer structure:
linear polymer - a polymer consisting of a single continuous
chain of repeat units
branched polymer - a polymer that includes side chains
of repeat units connecting onto the main chain of repeat units
(different from side chains already present in the monomers)
crosslinked polymer - a polymer that includes interconnections
between chains, either formed during polymerization (by choice
of monomer) or after polymerization (by adding a specific reagent)
network polymer - a crosslinked polymer that includes
numerous interconnections between chains such that the entire
sample is (or could be) a single molecule
configuration - the three-dimensional
structure of a polymer based on orientations that cannot be changed except
by
breaking of bonds (cis-trans, R&S); this definition is used
the same way as in organic chemistry, although there will be further
definitions and descriptions of configuration
conformation - the three-dimensional structure of a
polymer based on orientations that can be interconverted by simple
rotations of bonds; used the same way as in organic chemistry
3. Classification of polymerization reactions:
step-reaction polymerization - a polymerization mechanism
in which each reaction between monomers is a discrete and independent
step (e.g., formation of polyesters from diols plus diacids)
chain-reaction polymerization - a polymerization mechanism
in which monomers add rapidly to a relatively few reactive growth
sites (e.g., free radical chain polymerization of ethylene)
ring-opening polymerization - a polymerization mechanism
in which a cyclic monomer opens as it is attached in each growth
step (e.g., epoxy polymerizations)
4. Descriptions of polymer size:
degree of polymerization (DP) - the number of monomer
units incorporated into a polymer chain
monodisperse - describing a polymer sample consisting
of molecules ALL of which have the same chain length (an ideal,
hypothetical situation, except in natural macromolecules)
polydisperse - describing a polymer consisting of molecules
with a variety of chain lengths (and hence molecular weights)
- the real life situation for synthetic polymers
number-average molecular weight (Mn) - the average
molecular weight of a polydisperse polymer sample, averaged to
give equal statistical weight to each molecule; calculated as
Mn = SUM ( Mi Ni ) / SUM ( Ni )
weight-average molecular weight (Mw) - the average
molecular weight of a polydisperse polymer sample, averaged to
give higher statistical weight to larger molecules; calculated
as
Mw = SUM ( Mi^2 Ni ) / SUM ( Mi Ni )
EXAMPLE: consider a sample of 5 molecules of MW= 1, 2, 3, 4,
5
Mn = (1 + 2 + 3 + 4 + 5) / 5 = 3.00
Mw = (1 + 4 + 9 + 16 + 25) / (1 + 2 + 3 + 4 + 5) =
3.67
EXAMPLE: equal moles of polymer of MW 10,000 and MW 100,000
Mn = (0.5 *104) + (0.5 *105) / (1.0) = 55,000
Mw = (0.5 *108) + (0.5 *1010) / (0.5 *104) + (0.5 *105)
= 92,000
EXAMPLE: 90% polymer of MW 10,000 and 10% MW 100,000
Mn = (0.9 *104) + (0.1 *105) / (1.0) = 19,000
Mw = (0.9 *108) + (0.1 *1010) / (0.9 *104) + (0.1 *105)
= 57,000
5. Descriptions of polymer physical properties:
primary bonds - the covalent bonds that connect the
atoms of the main chain
secondary bonds - non-covalent bonds that hold one polymer
chain to another, including hydrogen bonding and other dipole-dipole
attractions; this term is used similarly in describing protein
structure
crystalline polymers - solid polymers with a high degree
of structural order and rigidity
amorphous polymers - polymers with a low degree of structural
order
semi-crystalline polymers - most polymers actually consist
of both crystalline domains and amorphous domains, with properties
between that expected for a purely crystalline or purely amorphous
polymer
glass - the solid form of an amorphous polymer, characterized
by rigidity and brittleness even though there is little order
on the molecular level
crystalline melting temperature (Tm) - temperature
at which a crystalline polymer converts to a liquid, or crystalline
domains of a semi-crystalline polymer melt (increased molecular
motion)
glass transition temperature (Tg) - temperature
at which an amorphous polymer converts to a liquid, or amorphous
domains of a semi-crystalline polymer melt
- a molecular interpretation of polymer melting/freezing behavior:
above Tm - polymer is liquid, although usually very viscous,
with free random motion of polymer chains
at Tm - if polymer can crystallize (orderly structure, slow
cooling), solid crystalline domains appear
at Tg - the remainder of the polymer chains solidify,
without much order, into a glassy state
below Tg - the polymer is a rigid solid, containing some
crystalline and some amorphous domains
polyethylene: Tg = -125°C , Tm = 137°C
(polyethylene is a flexible solid at room temp, consisting of
some solid crystalline domains and melted amorphous domains)
polystyrene: Tg = 100°C , Tm = 240°C
(polystyrene is a rigid, glassy solid at room temp)
high-density polyethylene - polyethylene created so as
to have a relatively high concentration of crystalline domains;
its properties are greater density, rigidity, and strength (e.g.,
gallon milk cartons)
low-density polyethylene - polyethylene created so as
to have mainly amorphous structure, with low density and great
flexibility (e.g., squeeze bottles, plastic bags)
thermoplastics (plastics) - polymers that undergo thermally
reversible interconversion between the solid state and the liquid
state
thermosets - polymers that continue reacting at elevated
temperatures, generating increasing numbers of crosslinks; such
polymers do not exhibit melting or glass transitions
liquid-crystalline polymers - polymers with a fluid
phase that retains some order
elastomers - rubbery, stretchy polymers; the effect
is caused by light crosslinking that pulls the chains back to
their original state
C. Nomenclature of Polymers
based on monomer source:
the most common method for naming addition polymers
(nearly always unambiguous)
e.g., polyethylene, poly(vinyl chloride) (PVC), poly(methyl methacrylate)
(PMMA), etc.
based on polymer structure:
the most common method for condensation polymers, since the
polymer typically contains different functional groups than the
monomers
e.g., poly(ethylene terephthalate) (Dacron), poly(hexamethylene
adipamide) (Nylon 66)
common or trade names:
frequently used for many polymers
IUPAC system:
identify repeat unit, starting with highest priority atoms
in chain and putting substituents on lowest number positions
e.g., -(OCHFCH2)- is the preferred repeat unit
(note that there are five other possible choices)
the correct name is poly[oxy(1-fluoroethylene)]
nomenclature is similar to normal IUPAC for organics,
but you need to know names for divalent groups:
-O- oxy
-NH- imino
-CH2- methylene
-CH2CH2- ethylene
(note: polyethylene is polymethylene by IUPAC)
-CH(CH3)- ethylidene
-CH(CH3)CH2- propylene
-CH2CH2CH2- trimethylene
-C(CH3)2- isopropylidene
-C6H4- phenylene (need o,m,p or numbers)
nomenclature examples:
-(OCH2)n- polyformaldehyde
poly(oxymethylene)
-(OOCC6H4COOCH2CH2)n- poly(ethylene terephthalate)
poly(oxycarbonyl-1,4-phenylenecarbonyloxyethylene)
D. Examples of polymer structures
essentially all of the functional groups possible in organic
chemistry have been incorporated into polymer structures, leading
to a tremendous variety of possible compounds and properties
1. Linear polymers (one main chain)
- all C-C bonds in the main chain:
saturated main chain (polyethylene, polypropylene)
unsaturation in the main chain (natural rubber, polyacetylene)
polar side groups (PVC, PMMA, Teflon)
- with O in the main chain:
polyformaldehyde (acetal)
epoxy polymers (PEO, PPO)
polyesters
polycarbonates
- with N in the main chain:
polyamines
polyamides (polypeptides)
polyurethanes
- with other heteroatoms in the main chain:
polysulfones
polysiloxanes (silicones)
polyphosphazenes
2. Linear polymers that include rings (cyclolinear polymers)
poly(p-phenylene)
polyaniline
cellulose
3. Branched polymers
generally modified form of the usual linear polymers
(e.g., branched polyethylene with 3° branch points)
multiple functionality (> 3)
at branch points lead to star polymers
starches are branched polysaccharides
4. Network Polymers
- loose networks - few crosslinks, insoluble but swellable
in solvents
(rubbers, soft contact lenses, polyacrylamide gels)
- dense networks (resins) - many crosslinks, rigid, insoluble
(epoxies, alkyd resins)
5. Copolymers
- homopolymer from a single monomer
- copolymer from two (or more) monomers
- terpolymer from three monomers
- multicomponent polymer from more than two monomers
- chain copolymerization (different monomers incorporated during
the growth of the polymer chain) can lead to:
- alternating copolymer - ABABABABABABABABABABABA
- block copolymer - AAAAAAAABBBBBBBBBBBBAAA
- random copolymer - BAABBAABABBABABBBAABBAB
- graft copolymer - branches of a different polymer are attached
to a polymer chain
AAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA
B
BBBBBBBBBBBBBBBBBBBBBBBBB
(generate reactive site on existing polymer chain, grow new chain)
E. Orientation and configuration in polymer chains
1. Head-to-tail orientation of monomer units predominates
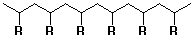
- polymer structure has substituents on alternate carbons because
each addition is preferred in the same direction
(revised Markovnikov Rule - form the more stable intermediate)
- a few sites of head-to-head will be present, either due to
occasional kinetic competition with the normal head-to-tail addition
(leads to one isolated reversed monomer)
or due to the termination step of recombination (leads to one
point where the order reverses, where the chains had been growing
in opposite directions)
2. Orientation and stereochemistry at double bonds
- conjugated dienes generally polymerize with 1,4 addition
(via allylic radical intermediate)
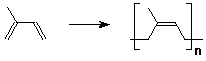
(isoprene monomer) -----> cis - natural rubber
(Note - natural rubber is head-to-tail: "The Isoprene
Rule")
3. Tacticity - configuration at chiral centers
CH2=CHX ---> -CH2-CHX-CH2-CHX-CH2-CHX-
...
- Note that achiral monomers can generate new chiral centers
in the polymer.
- However, chiral centers in most vinyl polymers are asymmetric
only by virtue of the differing chain lengths and end groups
in either direction.
- usually not designated as (R) or (S), only relative stereochemistry
isotactic - all chiral centers same
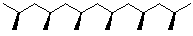
syndiotactic - chiral centers alternate
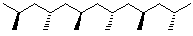
atactic - chiral centers random
- different polymerization methods lead to different types
and amounts of tacticity - requires that the previous position
on a growing chain influences the stereochemistry of the next
addition
- physical properties of the polymer depend on tacticity because
it influences the ease of crystallization
F. Polymerization Mechanisms
chain growth reactions - vinyl polymerization initiated
by radicals, cations, anions, or organometallic catalysts
step growth reactions - normal functional group reactions
of multifunctional monomers
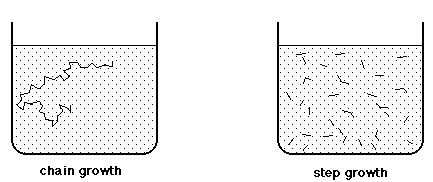
|
Chain Growth Polymerization
|
Step Growth Polymerization
|
| The only
growth reaction is addition of monomer to a growing chain with a reactive
terminus |
Reaction
can occur independently between any pair of molecular species |
The
reaction mixture consists of high
polymer and unreacted monomers, with very few actively growing
chains |
The
reaction mixture consists of oligomers of many sizes, in a statistically
calculable distribution |
| Monomer
concentration decreases steadily as reaction time increases |
Monomers
disappear early, in favor of low oligomers |
| High
polymer appears immediately, average molecular weight doesn't change
much as reaction proceeds |
Oligomers
steadily increase in size, polymer average molecular weight increases
as reaction
proceeds |
| Increased
reaction time increases overall product yield, but doesn't affect polymer
average molecular
weight |
Long
reaction times are essential to produce polymer with high average molecular
weight |
Radical polymerization
- initiation by radical sources ( peroxides, azo compounds)
- termination by coupling or disproportionation
- chain transfer leads to branching (e.g., H-abstraction in
a growing polyethylene chain)
Ionic Polymerization
- electron-rich alkenes can be polymerized by acids or Lewis
acids via cation intermediates
- termination by addition of nucleophile or loss of H+
- electron-deficient alkenes can be polymerized by bases or
nucleophiles via anion intermediates
- termination by addition of proton source (H2O)
Ziegler-Natta Catalysis
- heterogeneous (solid-phase) metal catalyst
- leads to highly linear polymers with minimal branching
- e.g., high-density polyethylene
- "living" polymerization - reactive chain terminus
remains active even after all monomer is used up (e.g., Ziegler-Natta
or anion intermediates in absence of terminating agents - will
continue to grow if "fed" more monomer)
G. Brief history of polymer chemistry
1833 - Berzelius - first uses term "polymeric" to
describe the relationship of ethylene (called olefiant gas, C2H4)
to butene (called oil of wine, C4H8) and higher homologs of empirical
formula CH2
1861 - Graham - measured diffusion
rates of various substances through parchment, identifying a number of unusually
slow materials
(both inorganic and organic), which he called "colloids" (Greek kolla - glue)
1921 - Staudinger - demonstrated
that many organic "colloids" were actually macromolecules, unlike
many inorganic colloids, which could be made to permeate by dilution (deaggregation);
prepared
many oligomers of formaldehyde diacetate
AcO(CH2O)nAc
n = 1-5 are liquids isolated by distillation
n = 8, 10, 12, 14, 15, 16, 17, 19 are solids isolated by fractional
crystallization
he compared mp, densities, and crystal structures of oligomers
with the polymer
(in 1937 succeeded in obtaining a single crystal structure of
polymer)
Staudinger established the following major principles:
1) atoms in polymers maintain their normal valences;
2) ends of polymers are normal functional groups;
3) end group analysis can lead to estimate of chain length
1930's - Carothers - performed and characterized numerous polymer
syntheses by condensation reactions, ultimately leading to the
introduction of the Nylon stocking in 1940
1940's - Flory, Mark, others - rapid expansion of polymer science,
including detailed studies of polymer structure and properties
and applications to a variety of materials of increasing commercial
value


![]()
![]()