Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2009 |
Quiz 27 |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2009 |
Quiz 27 |
![]()
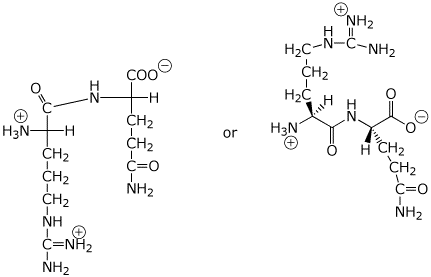
1. (4 points) Write an accurate structure for the dipeptide arginylglutamine (Arg-Gln), including correct stereochemistry and the expected ionization state at pH 7.
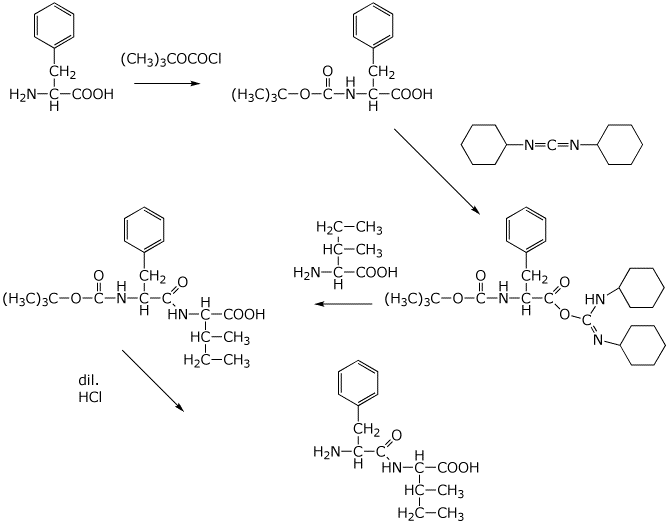
2. (3 points) Show the sequence of steps you would use to prepare the dipeptide Phe-Ile, starting from the amino acids, DCC as the coupling agent and BOC as the N-protecting group. Show the complete structures, but ignore stereochemistry.
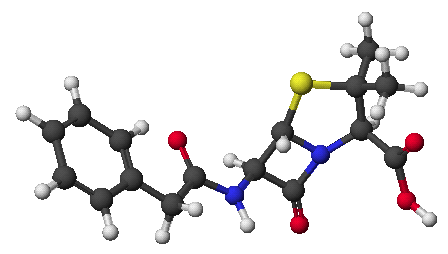
3. (3 points) Identify the unknown pentapeptide based on the following information.
Complete hydrolysis gives Ala, Leu, Lys, Trp, and Val
Carboxypeptidase releases Leu
Chymotrypsin also releases Leu
Trypsin releases the dipeptide TrpLeu
Sangerís reagent gives labeled Val
Val-Ala-Lys-Trp-Leu