Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2009 |
Quiz 20 |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2009 |
Quiz 20 |
![]()
1. (2 points) Give a complete name for each of the following:
a) 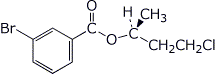
b) 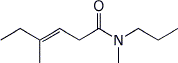
2. (2 points) Write the neutral tetrahedral intermediate and the product expected in each reaction below.
a) 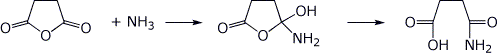
b) 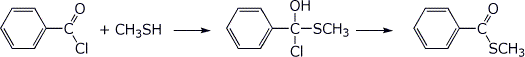
3. (2 points) Complete the reactions below by indicating the expected product.
a) 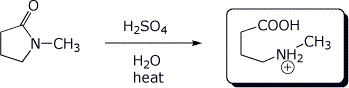
b)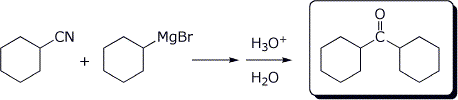
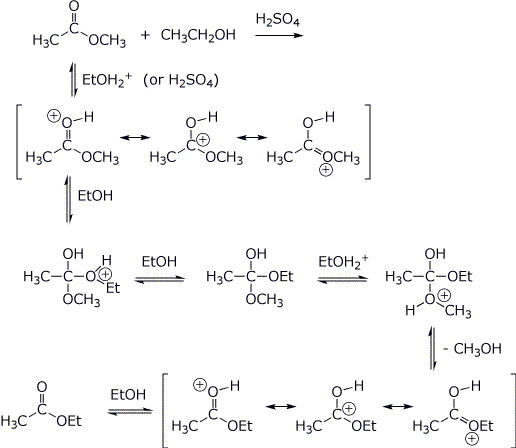
4. (4 points) Write a complete acid-catalyzed mechanism for the transesterification of methyl acetate to form ethyl acetate. Show all steps and all resonance forms for all intermediates involved. Electron-pushing arrows are optional.