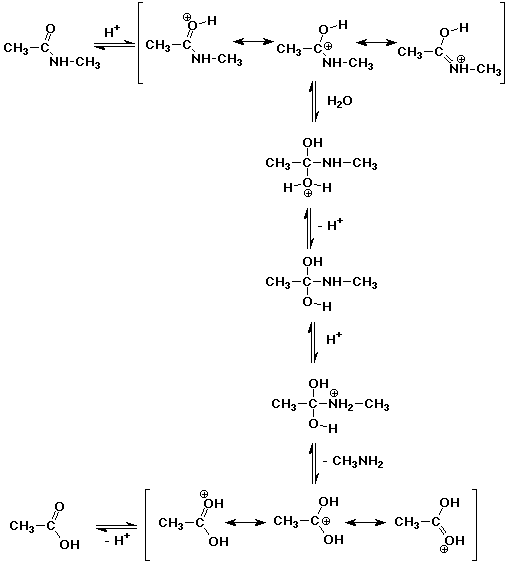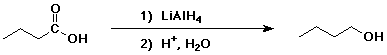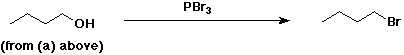|
|
Organic
Chemistry III
|
|
Professor
Carl C. Wamser
|
|
|
Chapter
20 Homework Answers
|

Carey, pages 908 - 915
Problems 20.22 - 40
1. Write complete names for each of the following:
a) 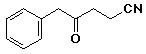
4-oxo-5-phenylpentanenitrile
b) 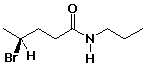
(S)-4-bromo-N-propylpentanamide
c) 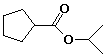
isopropyl cyclopentanecarboxylate
2. A typical protein linkage is an amide bond (also called a peptide bond), such
as might be found in N-methylacetamide. Proteins are typically hydrolyzed in
strong acid. Write a complete mechanism for the acid-catalyzed hydrolysis of
N-methylacetamide.
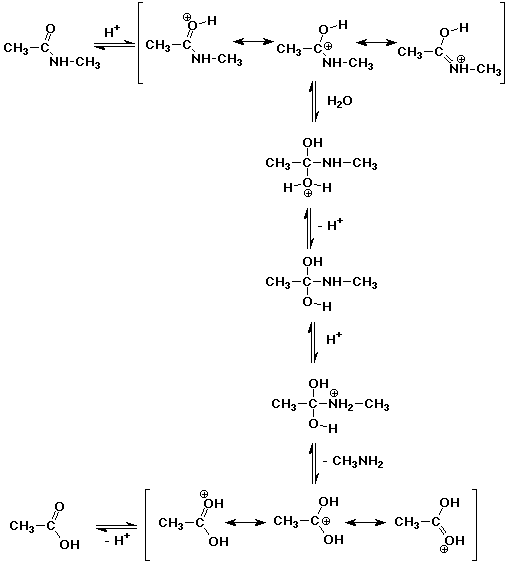
3. A typical fat has ester functional groups (most typically it is a triester
between three fatty acids and glycerol). Glycerol is 1,2,3-propanetriol, and
a fatty acid might be decanoic acid. Write the structure for the triester made
from these components.

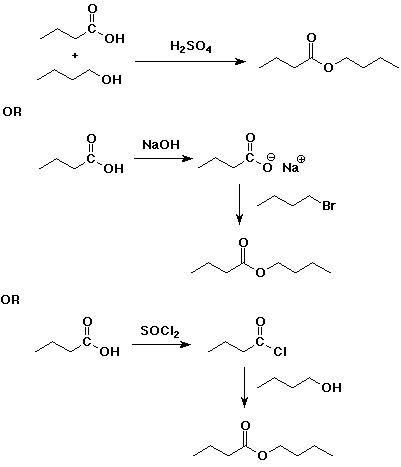
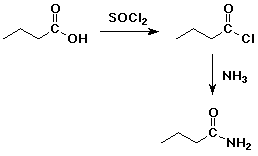
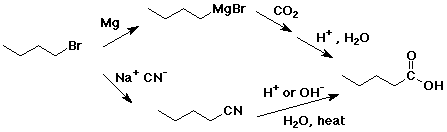
4. Using butanoic acid as the only organic starting material,
show how to prepare:
a) 1-butanol
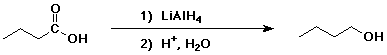
b) 1-bromobutane
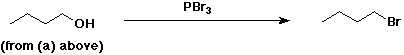
c) butyl butanoate
d) butanamide
e) pentanoic acid


![]()